This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
How interleukin-6 helps prevent allergic asthma and atopy by suppressing interleukin-2 signaling
The immune system has a biological telecommunications system—small proteins known as interleukins that send signals among the leukocyte white blood cells to control their defense against infections or nascent cancer. Interleukin-6, or IL-6, is one of these key mediators of inflammation, and it can, as needed, provoke the immune system into attack against pathogens.
However, imbalances of IL-6—too much or too little—can cause disease, even in the absence of infection. Excess IL-6 is central to the pathogenesis of inflammatory reactions like rheumatoid disease and cytokine storms, while mutations that interrupt IL-6 signaling are also harmful, causing allergic disorders known as atopy that affect the skin, airways or body, including atopic dermatitis, allergic airway inflammation and hyper-IgE Syndrome, or HIES.
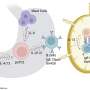
Loss of IL-6 signaling was known to cause an increase in inflammatory T helper 2, or Th2, cells. T helper cells act like generals, ordering other immune cells into action. Now, an unrecognized mechanism of how interrupted IL-6 signaling creates Th2 bias, as well as the specific role of IL-6 signaling in that process, has been described by Beatriz Léon, Ph.D., and colleagues at the University of Alabama at Birmingham. Their study is published in Cellular & Molecular Immunology.
"Understanding how IL-6 contributes to suppress allergic sensitization may offer new strategies to prevent atopic disease in patients with deficient IL-6 signaling," said Léon, an associate professor in the UAB Department of Microbiology.
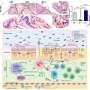
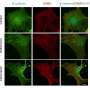
The UAB team used a mouse model of allergic airway inflammation, induced by house dust mite allergen, to drive a response where naïve CD4+ T cells differentiate into Th2 cells. In the model, mice received intranasal allergen for three days, and then were again exposed to allergen two weeks later to induce an allergic reaction. In the experiments, researchers used T cells of various genetic backgrounds and also used various biological inhibitors, such as antibodies against interleukins or against interleukin receptors.
Researchers found that IL-6 signaling in allergen-specific T cells was needed to suppress commitment to the harmful Th2 lineage. This mechanism is distinct, but complementary to, a previously described suppression mechanism involving interleukin-12 and the Tbet transcription factor.
The UAB team found that the harmful Th2 cell lineage commitment in their model required strong and prolonged signaling by interleukin-2, or IL-2, in cells unable to make or respond to IL-6. In wild type T cells, IL-6 shuts down IL-2 signaling early in T-cell activation to inhibit Th2 cell priming.
Mechanistically, IL-6 acts to upregulate SOCS3, the Suppression of Cytokine Signaling 3 protein, which is a negative-feedback inhibitor of certain cytokines that bind to receptors on the surface of cells. In the absence of SOCS3, binding of those cytokines activates the JAK/STAT internal signaling pathway that leads to altered gene expression. Cytokines is a general term for signaling proteins that include not only interleukins, but also interferons and growth factors.
SOCS3 is known to inhibit internal signaling by inhibiting the kinase activity of the JAK1 protein. In further support of their mechanism, Léon and colleagues found that a selective inhibitor of JAK1 was able to prevent Th2 cell priming in cells that did not receive IL-6 signaling, showing that IL-6 suppression of the harmful Th2 bias acted through inhibition of the JAK/STAT pathway.
The UAB group also found that IL-6 had to act early—the first two days after T-cell priming with house dust mite allergen—to turn off IL-2 signaling.
"Taken together, our data demonstrate that IL-6 signaling in allergen-specific T cells is essential for preventing Th2 development by counteracting IL-2-driven pro-Th2 signals," Léon said. "Our data provide insights into the immunological processes behind skewed Th2 responses in patients with defective IL-6 signaling or exposed to environmental factors that lead to decreased IL-6 synthesis."
More information: Holly Bachus et al, IL-6 prevents Th2 cell polarization by promoting SOCS3-dependent suppression of IL-2 signaling, Cellular & Molecular Immunology (2023). DOI: 10.1038/s41423-023-01012-1