This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Predicting the liver-tropism of AAV vectors based on pre-clinical liver models
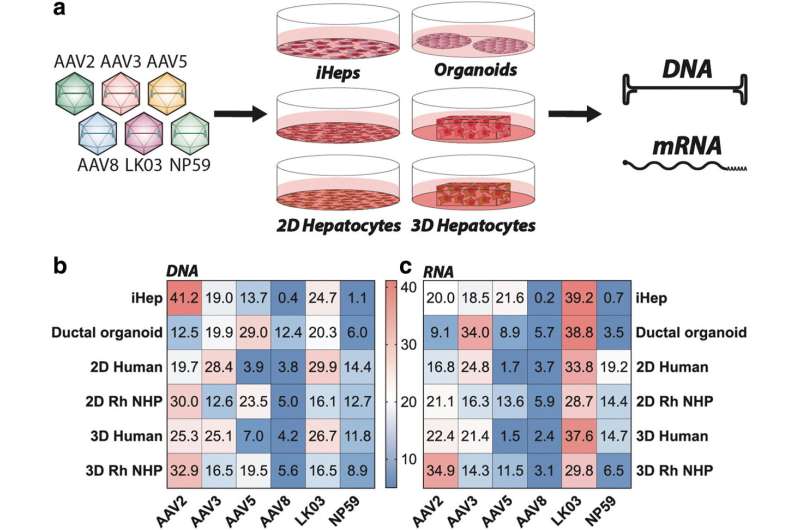
A new study reports the findings of the functional evaluation of six AAV vectors in 12 preclinical models of the human liver. The study, which aimed to uncover which combination of models is the most relevant for the identification of an AAV capsid variant for safe and efficient gene therapy to primary human hepatocytes, is published in Human Gene Therapy.
Leszek Lisowski, from The University of Sydney, and co-authors, note that robust, biologically- and clinically-predictive preclinical models of the safety and efficacy of liver-targeted AAV-based gene therapy are lacking. The investigators compared AAV-based gene transfer efficiency targeting the liver in 12 frequently used preclinical models, including in vitro models, such as hepatic cell lines, human-induced pluripotent stem cell (hiPSC)-derived hepatocytes, and adult stem cell-derived hepaltic organoids.
They focused primarily on ex vivo models, such as 2D and 3D primary non-human primates (NHP) and human hepatocytes cultures, and in vivo models, including murine and human hepatocytes in xenograft mice and NHPs.
"Even though a perfectly predictive preclinical model does not exist, our study shows that each model provides a unique insight into the vector function," stated the investigators.
"Based on our results and the fact that each model brings a unique perspective that adds to the overall functional evaluation of AAV vectors, we propose that multiple models should be used to paint a more complete picture and help us make the most informed decision as to which vector should be used in each clinical application."
"This study represents a highly innovative approach to combining data from multiple different experimental systems into an overall predictive model," says Editor-in-Chief Terence R. Flotte, MD, Celia and Isaac Haidak Professor of Medical Education and Dean, Provost, and Executive Deputy Chancellor, University of Massachusetts Chan Medical School.
More information: Adrian Westhaus et al, Assessment of Pre-Clinical Liver Models Based on Their Ability to Predict the Liver-Tropism of Adeno-Associated Virus Vectors, Human Gene Therapy (2023). DOI: 10.1089/hum.2022.188