This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A potential pathway to improved stroke recovery
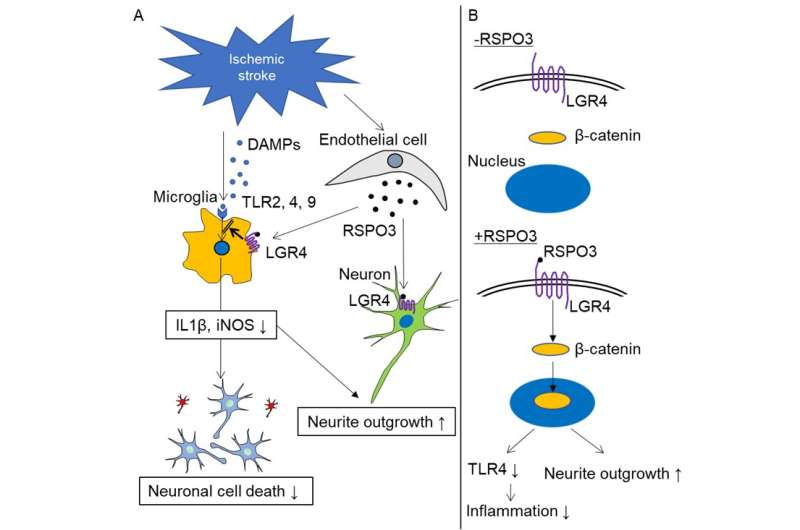
Ischemic stroke, caused by a blockage of blood flow to the brain, is a common cause of death and disability. Treatments are urgently needed to improve patient outcomes, because recovery currently depends largely on the timely injection of a blood clot-dissolving drug. Priorities for therapy include limiting inflammation at the ischemic site and rebuilding neuronal connections damaged by the stroke. However, a molecule that can achieve these therapeutic effects has remained elusive.
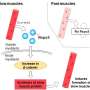
In a study to be published in Stroke, researchers from Osaka University provide new hope for patients. They have identified two proteins, R-spondin 3 (RSPO3) and LGR4, that trigger a cascade of reactions in cells (i.e., a signaling pathway) to reduce inflammation in the ischemic brain. RSPO3 and LGR4 also stimulate the growth of extensions from neurons, a process called neurite outgrowth.
"Previous studies showed that RSPO3 was beneficial in lung injuries caused by inflammation. We also knew that RSPO3 stimulates a signaling pathway, named the 'canonical Wnt pathway,' that promotes neurite outgrowth," explains Munehisa Shimamura, lead author of the study. "We wondered whether RSPO3 reduces inflammation and promotes neurite outgrowth after ischemic stroke."
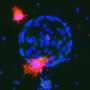
Previous studies have shown that RSPO3 and LGR4 are present in the same brain structures, and that RSPO3 activates LGR4 to stimulate the canonical Wnt pathway. The team from Osaka University localized RSPO3 in endothelial cells and LGR4 in microglia/macrophage cells and neurons in the ischemic brain.
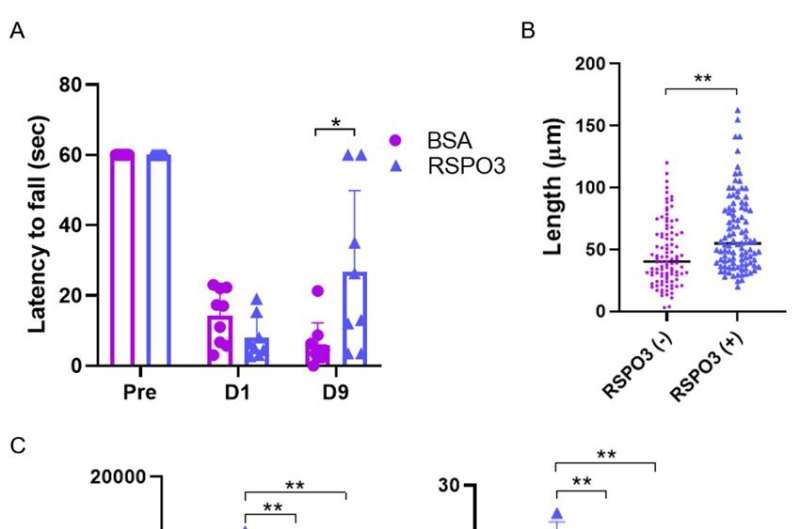
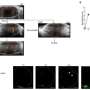
"Because of this close localization, RSPO3 could act on LGR4," explains Hironori Nakagami, a senior author of the study. "To test this hypothesis, we injected RSPO3 into the brains of mice 24 and 48 hours after ischemic stroke."
Remarkably, nine days after the stroke, mice that were injected with RSPO3 exhibited fewer sensory and motor deficits than mice injected with a control protein. The expression of pro-inflammatory factors was reduced, whereas signs of neurite outgrowth increased. How? The researchers found that RSPO3/LGR4 decreased the expression of TLR4, which is one of proteins essential for inducing inflammation.
These findings are particularly exciting because RPSO3 was given to mice one day after the stroke, suggesting a potential benefit to treatments in later stages of stroke. Thus, targeting RSPO3/LGR4 signaling is a promising lead for developing new therapies for ischemic stroke and improving patient outcomes.
More information: R-spondin 3/LGR4 axis is a novel inflammatory and neurite outgrowth signaling system in the ischemic brain in mice, Stroke (2023). DOI: 10.1161/STROKEAHA.122.041970