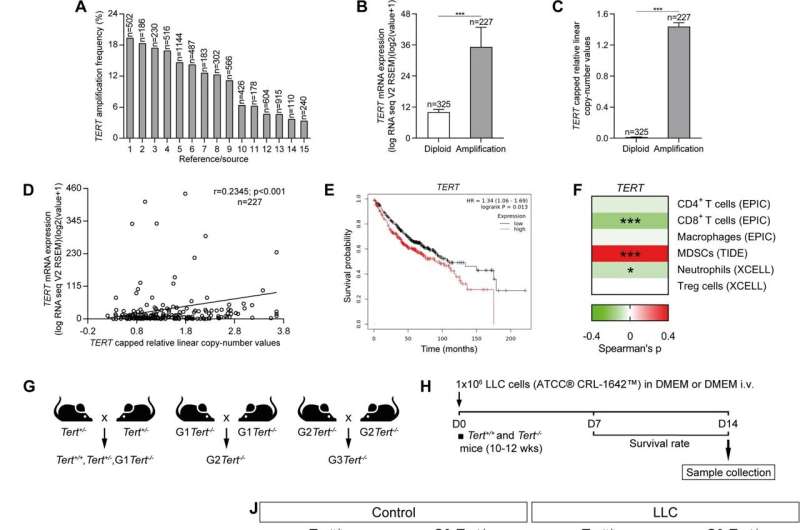
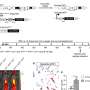
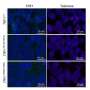
Increased amplification frequency, copy number values and mRNA expression of TERT in NSCLC patients, and reduced tumor implantation in TERT-deficient mice upon lung tumor induction. Amplification frequency (A), copy number values (B) mRNA expression levels of TERT (C) and Pearson correlation of mRNA expression with copy number values of TERT (D) in lung tissues from NSCLC patients, and survival probability in NSCLC patients with high and low TERT expression (E) obtained from the Kaplan–Meier Plotter database. F Correlation between the expression of TERT and immune infiltrates in NSCLC patients from the TCGA using the TIMER 2.0 database. G, H Generation of Tert+/+ and G3 Tert−/− mice and protocol for the induction of Lewis Lung Carcinoma (LLC). G Heterozygous Tert+/− mice were crossed to obtain Tert+/+ and G1 Tert−/− mice, and successive crosses between G1 Tert−/− and then G2 Tert−/− were set to generate G3 Tert−/− mice. H 1 ×106 Lewis cells suspended in 100 µl of DMEM or equal volume of DMEM (controls) were injected via the tail vein of 10–12 weeks old Tert+/+ and G3 Tert−/− male mice on day 0 (D0). An in vivo follow follow-up of survival was carried out until sample collection on day 14 (D14). Kaplan–Meier survival curves (I), representative images of LLC-challenged Tert+/+ and G3 Tert−/− lungs and controls (H&E) (J), and quantification of lung tumor area (K, L) and foci (M) in Tert+/+ and G3 Tert−/− mice. N Representative Telomeric repeat amplification protocol (TRAP) using S-100 lung extracts from LLC-challenged Tert+/+ and G3 Tert−/− mice and controls, where protein concentration is indicated. Extracts were treated (+) or not (−) with RNase as a negative control (exposition time: 48 h). An internal control (IC) for PCR efficiency was also included and arrows indicate the lane used for quantification. O Quantification of Telomerase activity in lung extracts from LLC-challenged Tert+/+ and G3 Tert−/− mice and controls expressed in arbitrary units (a.u). P Lung tissue mRNA expression levels of Tert normalized to 18S expression in Tert+/+ and G3 Tert−/− mice. Data are expressed as mean ± SEM (the number of mice is indicated in each case). ***p < 0.001 (Dunn–Sidak test for multiple comparisons and Mann–Whitney or unpaired t tests to compare 2 independent groups). Survival was assessed by the Kaplan–Meier analysis, using the log Rank (Mantel–Cox) test). Credit: Cell Death & Differentiation (2023). DOI: 10.1038/s41418-023-01149-6
Healthy cells can only divide a limited number of times during an organism's lifetime. In contrast, tumor cells are immortal: they proliferate indefinitely and uncontrollably, and this is the defining characteristic of cancer.
Researchers from the Telomeres and Telomerase Group at the CNIO (Spanish National Cancer Research Center), led by Maria Blasco, have studied for the first time the possibility of treating lung cancer by targeting the telomeres, the structures that protect the ends of chromosomes and whose condition determines the cell's ability to divide indefinitely.
The results, as explained by the researchers in the journal Cell Death & Differentiation, show that, indeed, targeting telomeres "might be an effective therapeutic strategy" against non-small cell lung cancer, which is responsible for much of the mortality in lung cancer patients. The work has Sergio Piñeiro as first author, recipient of a postdoctoral contract from the Spanish Association Against Cancer (AECC).
"Removing immortality from cancer cells is a therapeutic strategy that has not yet been exploited in the fight against cancer," says Maria Blasco.
Focus on the tumor microenvironment
Lung cancer is one of the leading causes of cancer death. The long-term ineffectiveness of current therapies and late diagnosis mean that only one in five patients survive more than five years. Specifically, non-small cell lung cancer is responsible for 85% of lung cancer-associated deaths.
The work now published focuses on the so-called tumor microenvironment, which is a set of cells and factors surrounding the tumor that plays a crucial role in the development of cancer and the response to therapies.
The researchers analyzed the impact of dysfunctional telomeres. Also, they studied the effect of telomerase deficiency on the cellular microenvironment of non-small cell lung tumors, telomerase being the enzyme that repairs telomeres.
Telomerase deficiency
Telomeres are protein structures located at the ends of chromosomes. At each cell division, telomeres shorten until, after a certain number of divisions, the shortening becomes excessive and the cell stops dividing. This happens in healthy cells, but not in most tumor cells.
Telomerase expression is reactivated in 90% of human tumors. Because of the action of telomerase, the telomeres in tumor cells maintain a minimum functional length, which allows them to divide indefinitely.
CNIO researchers studied what happened when they caused a telomerase deficit in the lung tumor microenvironment. They also deliberately damaged their telomeres, using the compound 6-thio-dG.
"It is the first time that the involvement of telomerase and dysfunctional telomeres in the lung tumor microenvironment has been investigated," explains Sergio Piñeiro, currently at the Spanish National Research Council (CSIC) in La Rioja.
Damaged telomeres hold back the tumor
Telomerase deficiency and dysfunctional telomeres slowed tumor progression. The researchers observed a reduction in tumor implantation and vascularization in the lung, while increasing the vulnerability of tumors to DNA damage and cell death. Tumor cell proliferation and inflammation were also decreased, and the anti-tumor response of the immune system was enhanced.
As the authors write in Cell Death & Differentiation, "We address by the first time the implication of TERT [telomerase] and dysfunctional telomeres in the lung tumor microenvironment. Our results demonstrate that targeting telomeres might be an effective therapeutic strategy in non-small cell lung cancer."
More information: Sergio Piñeiro-Hermida et al, Telomerase deficiency and dysfunctional telomeres in the lung tumor microenvironment impair tumor progression in NSCLC mouse models and patient-derived xenografts, Cell Death & Differentiation (2023). DOI: 10.1038/s41418-023-01149-6
Provided by The Spanish National Cancer Research Centre