The U.S. Food and Drug Administration said 12 cases of a neurological disorder have been reported following the application of a meningococcal vaccine.
The FDA said the 12 cases of Guillain-Barre Syndrome have been reported after administering of the vaccines, which are manufactured by French company Sanofi Pasteur, since October 2005, The Wall Street Journal reported Tuesday.
However, the FDA said sufficient evidence was not available to tie the vaccine to the disease and patients should continue to use it if instructed by their doctors.
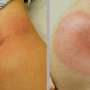
The vaccines are used to prevent meningococcal disease, which causes bacterial meningitis -- an infection that attacks the lining of the brain and spinal cord. The disease affects about 1-in-100,000 young people every year.
Copyright 2006 by United Press International