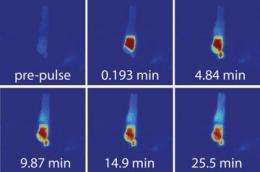
Calvert et al. measured protein diffusion through a sensory cilium. Credit: Calvert, P.D. 2010. J. Gen. Physiol. doi:10.1085/jgp.200910322.
A team of researchers led by Peter Calvert (SUNY Upstate Medical University) has, for the first time, measured the diffusion coefficient of a protein in a primary cilium and in other major compartments of a highly polarized cell. The study appears in the March issue of the Journal of General Physiology.
Transport of proteins to and from cilia is crucial for normal cell function and survival, and interruption of transport has been implicated in degenerative diseases and neoplastic diseases, such as cancer. Researchers believe that cilia impose selective barriers to the movement of proteins, but because of the narrow and complex structure of cilia—with diameters near or below the resolution of light microscopy—this hypothesis has been difficult to examine.
Using confocal and multiphoton microscopy, Calvert and his team—including William Schiesser (Lehigh University) and Edward Pugh (University of California, Davis)—measured the mobility of PAGFP (photoactivatable green fluorescent protein) in the connecting cilium (CC) of retinal rod photoreceptors in frogs, as well as in the subcellular compartments bridged by the CC. In addition, the team measured the overall time for the protein concentration to equilibrate within and between compartments.
The results establish that the CC does not pose a major barrier to protein diffusion within the rod cell, but that the axial diffusion in each of the rod's compartments is substantially delayed relative to that in aqueous solution.
More information: Calvert, P.D. 2010. J. Gen. Physiol. doi:10.1085/jgp.200910322
Provided by Rockefeller University