The Food and Drug Administration has approved a new drug from Roche to help treat patients with a type of cancer of the blood and bone marrow.
The agency cleared Gazyva to fight chronic lymphocytic leukemia in combination with chemotherapy in patients who haven't previously been treated for the disease.
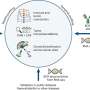
Gazyva works by killing cancer cells and encouraging the immune system to fight them.
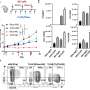
The FDA approved the drug based on a study showing Gazyva plus chemotherapy was superior to chemotherapy alone at slowing the progress of the disease. Patients treated with Gazyva had median survival of 23 months before death, relapse or worsening of their disease. That compares with 11.1 months for the chemotherapy patients.
Gazyva will be marketed by Roche's Genentech unit, based in South San Francisco.
© 2013 The Associated Press. All rights reserved.