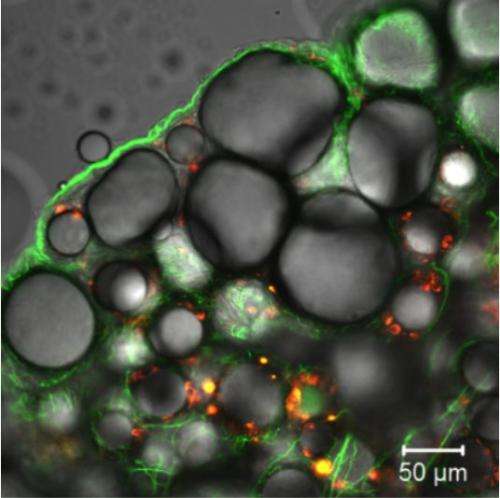
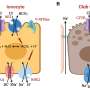
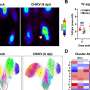
The confocal micrograph shows RFP (Red Fluorescence Protein) expression in obese adipocytes and vascular endothelial cells after an injection of ATS-9R/RFP oligopeptide complex. ATS-9R is capable of delivering a plasmid RFP specifically to obese fat tissues followed by RFP expression in adipocytes. This provides a basis for our hypothesis that ATS-9R can deliver an anti-obese shRNA, shFABP4 (Fatty Acid Binding Protein 4) that reduces the lipid accumulation in adipocytes, to obese fat tissues where shFABP4 has to locate to exert its anti-obesity activity. Green fluorescence (FITC) indicates the location of ATS-9R and Red (RFP) fluorescence represents RFP expression. Credit: Yong-Hee Kim
A team of researchers working in South Korea, has developed a technique for delivering a therapeutic gene to fat cells, causing the fat cells to function less efficiently, thereby reducing weight in test mice. In their paper published in Nature Materials, the team describes how they developed their technique, their results and problems they have yet to overcome.
As the researchers note, most research into finding a drug that can cause weight reduction in people with obesity issues has centered around appetite reduction. As the researchers note, thus far, this approach has not proved feasible—those that work to some degree cause serious side effects. Thus, the researchers with this new effort chose to direct their efforts directly at fat cells.
The research involved two basic steps, the first was to develop a carrier that would not only carry a gene therapy to fat cells, but would bind to them, allowing the therapy the opportunity to do its work. They developed one by putting together a short peptide with a gene sequence that targets fat cells. The result was a nine-amino-acid adipocyte targeting sequence that not only was able to bind to fat cells, but allowed for entering the cell nuclei—the carrier binds with fat cells by sticking to a protein found on their surface. Next, the researchers loaded the carriers with short hairpin RNA which prior research has shown is able to silence the fatty acid protein-4—injecting the carrier and its cargo into fat test mice that continued to be fed a high-fat diet showed weight loss in excess of 20 percent along with a higher tolerance for glucose, improved insulin sensitivity and an overall increase in their metabolic profile—all positive outcomes.
Unfortunately, while the initial results were promising, the researchers found that the carriers also bound to fat cells in places that were not desirable, such as in the liver or kidneys, which would likely lead to health problems. Thus, much more research will need to be done before the team looks into whether the same type of delivery system might be feasible for human beings.
More information: Oligopeptide complex for targeted non-viral gene delivery to adipocytes, Nature Materials (2014) DOI: 10.1038/nmat4092
Abstract
Commercial anti-obesity drugs acting in the gastrointestinal tract or the central nervous system have been shown to have limited efficacy and severe side effects. Anti-obesity drug development is thus focusing on targeting adipocytes that store excess fat. Here, we show that an adipocyte-targeting fusion-oligopeptide gene carrier consisting of an adipocyte-targeting sequence and 9-arginine (ATS–9R) selectively transfects mature adipocytes by binding to prohibitin. Injection of ATS–9R into obese mice confirmed specific binding of ATS–9R to fat vasculature, internalization and gene expression in adipocytes. We also constructed a short-hairpin RNA (shRNA) for silencing fatty-acid-binding protein 4 (shFABP4), a key lipid chaperone in fatty-acid uptake and lipid storage in adipocytes. Treatment of obese mice with ATS–9R/shFABP4 led to metabolic recovery and body-weight reduction (>20%). The ATS–9R/shFABP4 oligopeptide complex could prove to be a safe therapeutic approach to regress and treat obesity as well as obesity-induced metabolic syndromes.
Journal information: Nature Materials
© 2014 Phys.org