Credit: Chris Crocker/Albert Einstein College of Medicine
To stay healthy, neurons must prevent protein aggregates and defective organelles such as mitochondria from accumulating inside them. We now know that an animal species has found a solution to its neuronal trash problem—one that might also be present in humans and lead to neurodegenerative disease if it becomes dysfunctional.
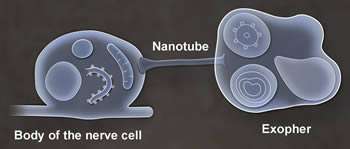
Researchers studying the roundworm C. elegans have discovered that neurons in adult worms possess a previously unrecognized garbage-removal mechanism: The neurons expel large (4- micron diameter) membrane-bound vesicles (dubbed "exophers") that are filled with clumped protein and damaged cellular organelles including mitochondria. The findings are described in a paper published in today's issue of Nature. One of the paper's senior authors is David H. Hall, Ph.D., professor in the Dominick P. Purpura Department of Neuroscience.
The researchers observed that inhibiting other avenues of protein degradation—autophagy and proteasomal digestion, for example—enhanced exopher production. And when roundworm neurons were induced to express high levels of neurotoxic huntingtin protein, they produced significantly more exophers than did neurons in control worms. Inducing neurons to express another toxic protein (amyloid-forming human Alzheimer's disease fragment) yielded similar results.
Importantly, neurons stressed by toxic proteins seem to function better after they generate exophers. For example, several strains of roundworm express altered proteins that progressively impair touch sensation. At midlife in these strains, the touch sensitivity of a particular touch-detector neuron was enhanced in worms that produced exophers earlier in their lives compared with worms that had not.
After discovering that exophers can also expel mitochondria, the researchers found they could trigger exopher production by stressing, damaging or otherwise impairing mitochondrial quality. For example, increased production of neuronal exophers was observed in roundworm strains in which either of two genes involved in mitochondrial maintenance were rendered defective.
What is the fate of exophers and their trash after neurons jettison them? Data supported by electron microscopy suggested that at least some of the material is degraded by neighboring cells of the worm's hypodermis (the cell layer that secretes its outer cuticle layer). But a portion of the exopher material entered the worm's body cavity and was scavenged by distant cells. If human neurons possess the equivalent of exophers, the researchers note, then this transfer of potentially toxic material could have implications for neurological disease.
Recent findings indicate that mammalian neurons can expel protein aggregates associated with Alzheimer's, Parkinson's and prion disease. Once outside the neuron, these aggregates can be taken up by other cells—possibly the way disease damage spreads in the brain.
"We propose that exophers are components of a conserved mechanism that constitutes a fundamental, but formerly unrecognized, branch of neuronal proteostasis [protein homeostasis] and mitochondrial quality control, which, when dysfunctional or diminished with age, might actively contribute to pathogenesis in human neurodegenerative disease and brain aging," the researchers concluded.
More information: Ilija Melentijevic et al. C. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress, Nature (2017). DOI: 10.1038/nature21362
Journal information: Nature
Provided by Albert Einstein College of Medicine