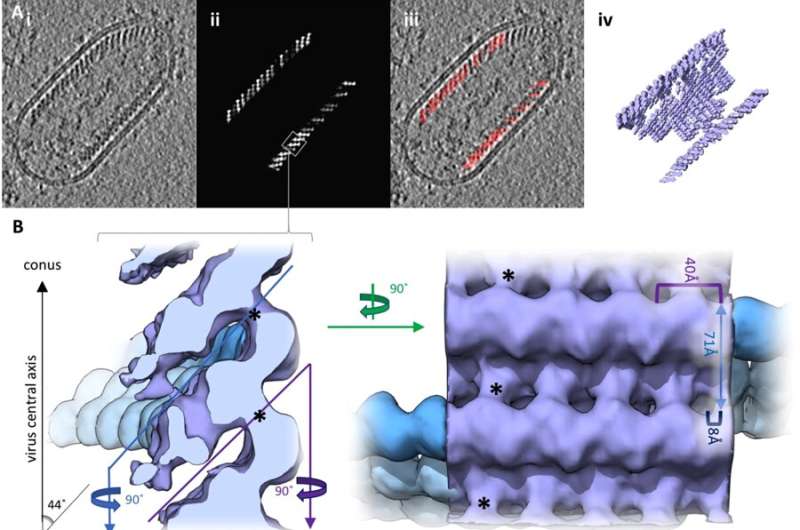
Reconstruction of the RABV RNP complex. (A) Slice through a tomogram (i) and the respective slice of a volume generated by plotting the final average back on the position of each subtomogram in a given tomogram (ii). The boxed area indicates an area equivalent to the average shown in B. (iii) shows an overlay of (i) and (ii) and a 3D representation of (ii) is shown in (iv). (B) Side and top view of the final average, filtered to 15 Å. The angular deviation of the helical turns from the central axis of the virus, as well as the distances between turns (light blue), subsequent units on one turn (purple) and shift between units on neighbouring turns (dark blue) are indicated. The densities connecting neighbouring helical turns are marked by black asterisks. The progression of the RNP from the average is depicted in blue for clarification. (C) Front (facing the particle conus) and back facing view of a helical turn. N-protein monomers (PDB: 2GTT, in purple and cyan) were docked in the electron density map. RNA resolved in the crystal structure is shown in dark blue and neighbouring nucleotides are connected by dark blue dotted lines. (D) Histogram depicting the distance to the nearest neighbour of each model point. (E) Comparison of the geometry between neighbouring N subunits in the crystal structure (yellow) and the electron density map (purple) with a reference subunit in cyan. (F) Plot of the FSC. Source: Christiane Riedel et al. Cryo EM structure of the rabies virus ribonucleoprotein complex, Scientific Reports (2019). DOI: 10.1038/s41598-019-46126-7
A research team of the Institute of Virology of the University of Veterinary Medicine Vienna has achieved a major breakthrough in exploring the rabies virus: for the first time, researchers were able to exactly depict the structure of the RNP of this virus that is highly dangerous to terrestrial mammals.
The rabies virus (RABV) (genus Lyssavirus, family Rhabdoviridiae, order Mononegavirales) is the primary causative agent of rabies in terrestrial mammals. Human beings are also massively affected by this mortal danger. The WHO estimates the annual human death toll to be more than 55,000.The RABV particle consists of a cell derived membrane, in which multiple copies of thesurface glycoprotein areanchored, and a helical ribonucleoprotein (RNP), which forms a conical tip at one end.
Although the individual components of the RNP had already been known, the exact structure of an intact RABV-RNP complex had not yet been identified. Using cryoelectron tomography, an imaging procedure allowing for the three-dimensional representation of the smallest biological structures, and a subsequent computer-assisted analysis by subtomogram averaging, a research team of the University of Veterinary Medicine Vienna around Christiane Riedel, the study's first author, and Till Rümenapf, the study's last author, has now succeeded in doing exactly that.
Two viruses as unlike siblings: similar structures, different appearances
The virus structure consists of a right-handed helix, with the 3'-terminal end of the genome located in the RNP cone, as observed in the related Vesicular stomatitis virus (VSV). Vesicular stomatitis is a virus disease with a mild course that mainly affects hoofed animals and may cause flu-like symptoms in humans. "In RABV, interactions between M- and N-proteins are responsible for the connection of neighbouring helical turns, while M-M interactions have been described for VSV. This results in a greater distance between the helix turns compared to VSV and, thus, in a shallower angle between the individual RNP turns and the central virus axis. This shows a surprising structural variability of the RNP when comparing VSV and RABV, although the crystal structures of the individual components that had already been determined, i.e. the N- and M-proteins, are highly homologous. To put it differently: Although the individual components of RABV and VSV are very similar, there are significant differences in the architectures of the RNPs of the two viruses," explains Christiane Riedel.
More information: Christiane Riedel et al. Cryo EM structure of the rabies virus ribonucleoprotein complex, Scientific Reports (2019). DOI: 10.1038/s41598-019-46126-7
Journal information: Scientific Reports
Provided by University of Veterinary Medicine—Vienna