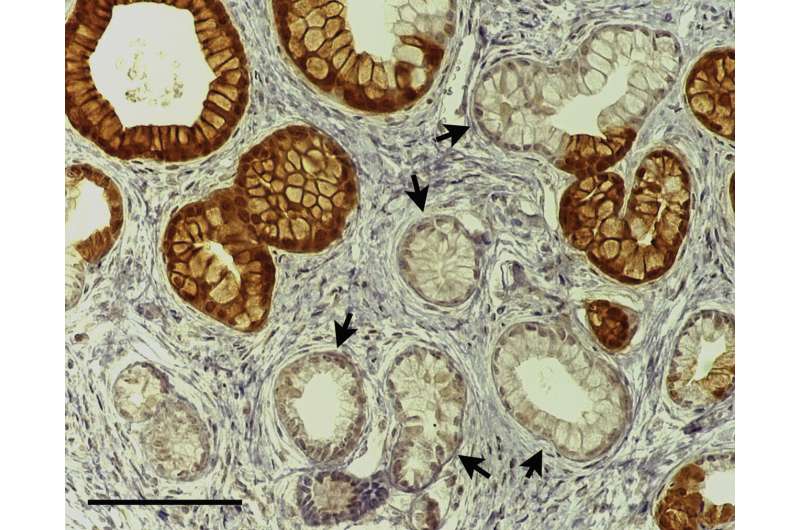
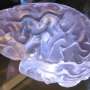
Figure 1Kras mutant phenotype occurs in EYFP+ and in EYFP– cells in Ptf1aCreERTM LSL-KrasG12D R26REYFP mice, reflecting broader recombination of the LSL-KrasG12D allele than of the R26REYFP allele. Note the PanIN-like lesions with tall columnar morphology and basal nuclei, some of which are positive for EYFP (brown) and some of which are negative (arrows). DOI: https://doi.org/10.1016/j.jcmgh.2019.07.001
Chronic pancreatitis (CP) is a predisposing condition for pancreatic ductal adenocarcinoma (PDAC), the most common and deadly cancer of the pancreas. However, the link between CP and PDAC is not known.
To explore this connection, Anna Means, Ph.D., and colleagues used a mouse model in which a pancreatic duct obstruction was induced in conjunction with an activating mutation in the KRAS oncogene, as is commonly seen in human disease.
Reporting in the journal Cellular and Molecular Gastroenterology and Hepatology, the researchers found that CP promoted KRAS-initiated cancer in the ductal cells of the pancreas but not in the acinar cells which synthesize, store and secrete digestive pro-enzymes.
While acinar cells upregulate the tumor suppressor gene p53 and its target gene p21, thereby resisting neoplastic changes, ductal cells lack a p53/p21 response and become proliferative after obstructive CP, thereby contributing to PDAC progression.
These findings uncover a mechanism by which inflammation and intrinsic cellular programming synergize for the development of PDAC.
More information: Chanjuan Shi et al. Differential Cell Susceptibilities to Kras in the Setting of Obstructive Chronic Pancreatitis, Cellular and Molecular Gastroenterology and Hepatology (2019). DOI: 10.1016/j.jcmgh.2019.07.001
Provided by Vanderbilt University