Credit: CC0 Public Domain
Early treatment with anti-VEGF injections slowed diabetic retinopathy in a clinical study from the DRCR Retina Network (DRCR.net). However, two years into the four-year study its effect on vision was similar to standard treatment, which usually begins at the onset of late disease. The intermediate findings published today in the JAMA Ophthalmology. The study was supported by the National Eye Institute (NEI), a part of the National Institutes of Health.
"While it is possible that preventive injections of anti-VEGF drugs may help protect vision in the longer-term, we saw no effect on vision at two years," said Raj Maturi, M.D., Indiana University, the protocol chair for the study. "These 2-year results suggest that close monitoring and routine treatment when complications develop are key to preventing vision loss from diabetic retinopathy."
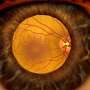
An estimated 30 million Americans have diabetes, which can cause blood vessel abnormalities, including the growth of new blood vessels in the eye, called diabetic retinopathy. In the early stages of diabetic retinopathy, called non-proliferative diabetic retinopathy (NPDR), changes in the eye's blood vessels are visible to clinicians but generally do not affect sight. In the advanced stages, people can develop proliferative diabetic retinopathy (PDR), where retinal blood vessels grow abnormally, and/or diabetic macular edema (DME), where fluid leaks out of the retinal blood vessels. Both can lead to vision loss and blindness. Treatment, such as with anti-VEGF drugs, can slow or prevent vision loss in people with PDR or DME, as long as treatment occurs promptly.
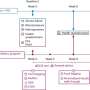
In this study, participants with NPDR were randomly assigned at baseline to receive either injections of Eylea (aflibercept) or a sham injection. They were examined at one, two, and four months, and then every four months for two years, receiving Eylea or sham injection at each visit. The researchers tracked their visual acuity and the severity of their diabetic retinopathy. If disease progressed, regardless of whether they were in the treatment or sham group, participants were given Eylea more frequently as is given in standard practice. If their condition did not improve with additional anti-VEGF treatment, participants could be given treatments such as laser photocoagulation or surgery if necessary.
The study included 328 participants (399 eyes). In two years, the rate of PDR development was 33% in the control group, compared with 14% in the treatment group. Likewise, the rate of development of DME affecting vision was 15% in the control group, compared with 4% in the treatment group. However, loss of visual acuity was essentially the same between the two groups at 2 years, suggesting that standard treatment at the appearance of PDR or DME affecting vision is sufficient to prevent further vision loss at this time point.
"We have a really good treatment for these diseases, so we can manage vision complications that may arise as disease progresses for many eyes," said Adam Glassman, Jaeb Center for Health Research, director of the DRCR.net coordinating center. "When evaluating new preventative treatment strategies, it is important to compare them directly to the standard treatment after disease worsens, as we have done in this study."
"Although we did not see any difference in visual outcomes at two years, the four-year follow-up is going to be very important," said Jennifer Sun, M.D., M.P.H., Joslin Diabetes Center, Harvard Medical School, chair of Diabetes Initiatives for the Network. "We look toward the four-year data to see whether reducing rates of diabetic retinopathy worsening will lead to long-term improvement in visual outcomes."
More information: Maturi RK, Glassman AR, Josic K, Antoszyk AN, Blodi BA, Jampol LM, Marcus DM, Martin DF, Melia M, Salehi-Had H, Stockdale CR, Punjabi OS, and Sun JK, for the DRCR Retina Network. "A Randomized Trial of Intravitreous Anti-VEGF for Prevention of Vision Threatening Complications of Diabetic Retinopathy (Protocol W)." JAMA Ophthalmology. March 30, 2021.
Journal information: JAMA Ophthalmology
Provided by National Eye Institute