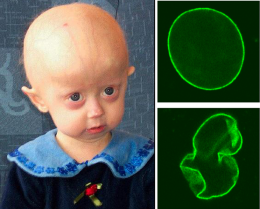
Hutchinson-Gilford Progeria Syndrome. HGPS is a childhood disorder caused by mutations in one of the major architectural proteins of the cell nucleus. In HGPS patients the cell nucleus has dramatically aberrant morphology (bottom, right) rather than the uniform shape typically found in healthy individuals (top, right). Image: PLoS Biology Vol. 3/11/2005, e395 (via Wikipedia)
(Medical Xpress) -- A new study published in the journal Science Translational Medicine shows that rapamycin and its derivative everolimus, which is currently used to treat cancer and transplant rejections, may work to reverse the aging effects seen in children with Hutchinson-Gilford progeria syndrome, better known as simply progeria.
Progeria is a rare and fatal genetic disease that affects children and causes rapid aging. It is caused by a defect that affects the processing of the protein lamin A which is responsible for shaping a cell’s nucleus. The cells instead produce a large amount of progerin, or an abnormal form of lamin A, which causes a disruption to the cells. A buildup of this progerin in the body affects the normal development of tissues in the body. The disease is usually fatal by age 12.
Led by Francis S. Collins, the director of the National Institutes of Health, a team of researchers looked at everolimus and its effects on the mutant protein progerin. Progerin is a protein that is found in everyone as they age, not just in children with progeria. Their original research showed that everolimus was able reduce progerin in healthy individuals and showed the ability to prolong cell life.
The research shows that the everolimus seems to boost the cells internal recycling system to eliminate the progerin as much as 50 percent. The researchers believe that these findings provide enough data to begin clinical trials on children with progeria. In the laboratory, the drug was administered to cells from progeria patients and the progerin protein in the cells disappeared.
In November of 2010, the FDA approved Afinitor, a brand-name everolimus tablet, for the treatment of benign brain tumors. The approval was based on a trial conducted at the Cincinnati Children’s Hospital Medical Center, so the drug has been tested on children before. The hope is they can get quick approval to begin a clinical trial on children with progeria.
This is not the first drug being tested on children with progeria, though the other three drugs currently in trial work in a different way. They are cancer-fighting drugs called farnesyltransferase inhibitors that may work to prevent the production of progerin. It is the hope of Collins that together these two drugs could provide a significant treatment for children with progeria.
More information:
Rapamycin Reverses Cellular Phenotypes and Enhances Mutant Protein Clearance in Hutchinson-Gilford Progeria Syndrome Cells, Sci Transl Med 29 June 2011:
Vol. 3, Issue 89, p. 89ra58. DOI:10.1126/scitranslmed.3002346
ABSTRACT
Hutchinson-Gilford progeria syndrome (HGPS) is a lethal genetic disorder characterized by premature aging. HGPS is most commonly caused by a de novo single-nucleotide substitution in the lamin A/C gene (LMNA) that partially activates a cryptic splice donor site in exon 11, producing an abnormal lamin A protein termed progerin. Accumulation of progerin in dividing cells adversely affects the integrity of the nuclear scaffold and leads to nuclear blebbing in cultured cells. Progerin is also produced in normal cells, increasing in abundance as senescence approaches. Here, we report the effect of rapamycin, a macrolide antibiotic that has been implicated in slowing cellular and organismal aging, on the cellular phenotypes of HGPS fibroblasts. Treatment with rapamycin abolished nuclear blebbing, delayed the onset of cellular senescence, and enhanced the degradation of progerin in HGPS cells. Rapamycin also decreased the formation of insoluble progerin aggregates and induced clearance through autophagic mechanisms in normal fibroblasts. Our findings suggest an additional mechanism for the beneficial effects of rapamycin on longevity and encourage the hypothesis that rapamycin treatment could provide clinical benefit for children with HGPS.
© 2010 PhysOrg.com