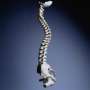
Preoperative cyanoacrylate sealant (InteguSeal) application does not reduce the risk of surgical site infection for patients undergoing scoliosis surgery, according to a study published online July 18 in Spine.
(HealthDay) -- Preoperative cyanoacrylate sealant (InteguSeal) application does not reduce the risk of surgical site infection for patients undergoing scoliosis surgery, according to a study published online July 18 in Spine.
Eric Dromzee, M.D., from the Université Pierre et Marie Curie in Paris, and colleagues randomly assigned 56 patients with neuromuscular or adolescent idiopathic scoliosis (AIS) to receive either a sterile, film-forming cyanoacrylate liquid application (InteguSeal) or no application. Infection occurrence was compared in the two groups.
The researchers found that the neuromuscular patients had more fused levels, increased intraoperative bleeding, and longer intraoperative time than AIS patients. Three deep and three superficial early postoperative infections of the posterior approach occurred in six patients. Five of the six infections occurred in patients treated with InteguSeal, but non-parametric statistical tests (Fischer exact test) showed no significant correlation (P = 0.096) between early postoperative infection occurrence and the use of InteguSeal. After local wound debridement and treatment with antibiotics all six cases had favorable outcomes.
"If microbial sealant may thus be a useful addition to a multimodal approach to minimize surgical site infection, there is currently insufficient evidence as to whether the use of microbial sealants reduces the risk of surgical site infection in people undergoing scoliosis surgery," the authors conclude.
More information:
Abstract
Full Text (subscription or payment may be required)
Copyright © 2012 HealthDay. All rights reserved.