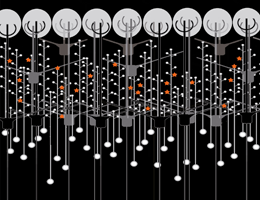
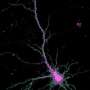
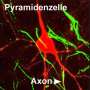
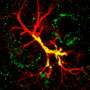
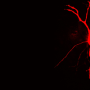
Schematic depiction of a network in the olfactory bulb showing local activation of single spines (red stars). Credit: Regensburg University
Nerve cells use a much larger repertoire of data-processing structures than previously thought. Research at LMU and in Regensburg shows that the so-called spines on the dendritic processes of neurons are able to process stimuli locally.
A recent study by teams of neurobiologists based at LMU and Regensburg addresses the role of the short protrusions called "spines" that form on the dendrites of nerve cells in the mammalian olfactory bulb. Dendrites serve as receptors for signals delivered by the so-called axons of other neurons. As the researchers now report in in the journal Neuron, their findings show that each of the many spines on the dendritic "trees" on these nerve cells is capable of processing incoming signals locally and independently of the activation state of the rest of the cell. In other words, each dendritic spine operates as an independent computational unit.
Nerve cells communicate by means of electrical signals. Membrane processes called dendrites act as antennas and processing sites for incoming signals. The overall result of this processing or neural computation then determines the cell's response, in the form of the so-called action potential that is induced in the axon and transmitted to other neurons. Moreover, most of the nerve cells in the brains of mammals carry smaller protrusions on their dendrites. These are the spines, which constitute the functional contacts or synapses with other neurons. But how spines actually work has been unclear, although some theorists had proposed in the 1960s that they might provide localized electrical amplification for incoming synaptic signals. Such a possibility would also markedly extend the capacity of nerve cells to process the information they receive.
The new study focused on the spines of specialized neurons called granule cells found in the olfactory bulb. Professor Veronica Egger and her colleagues at Regensburg University were able, for the first time, to show that localized signal amplification indeed occurs within the spines of these cells. In collaboration with theorists led by LMU's Professor Andreas Herz and Dr. Martin Stemmler, who are also affiliated with the LMU Bernstein Center for Computational Neuroscience in Munich, they went on to identify the underlying mechanism – the ability of a single spine to evoke an action potential. This in effect means that each spine behaves like a mini-neuron.
In the case of the granule cells in the mammalian olfactory bulb, this local amplification of the incoming signal is of particular significance, because they lack dedicated axons. Instead, their dendritic spines not only receive signals transmitted by other neurons, they can themselves initiate and propagate signals just like axons do. Similar "reciprocal synapses" that serve as functional interfaces between neurons are also found in other areas of the central nervous system, such as the retina and the thalamus.
The amplification mechanism defined in the new study is a consequence of the fact that the spines formed by the granule cells carry voltage-dependent ion channels similar to those normally found in the axonal membrane. This enables each spine to perform neural computations independently of the activation state of the rest of the granule cell. The members of the Regensburg and Munich groups expect that their findings will also prove to be valid for other types of reciprocal synapses in mammalian nervous systems. At all events, their results demonstrate that information processing in individual nerve cells is far more complex than previously assumed.
More information: Neuron, DOI: 10.1016/j.neuron.2014.12.051
Journal information: Neuron
Provided by Ludwig Maximilian University of Munich