(Medical Xpress)—A team of researchers from several instantiations in the U.S. has found a connection between two defective genes in humans and the messages that are sent from a type of good bacteria and Irritable Bowel Syndrone (IBS). In their paper published in the journal Science, the team describes their studies with mice, isolated human cells and a type of bacteria found in the human gut called Bacteroides fragilis.
Prior research has shown that B. fragilis is a "good" type of bacteria, which means if offers benefits rather than causing problems—other research has found that people with Crohn's disease have defects in two particular genes, NOD2 and ATG16L1 which cause problems by instigating, or not preventing, inflammation in the bowel. But what has not been clear is how the defective genes cause the disease. In this new effort, the researchers have identified a major part of the process.
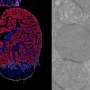
By genetically altering mice to have the human defective NOD2 and ATG16L1 genes, and then watching carefully what occurred, the team was able to see that B. fragilis actually helped to prevent the inflammation associated with Crohn's disease and IBS—after introduction into the gut of mice without the defective genes, they were able to see that the bacteria sent out little packets called outer membrane vesicles (OMVs) that contained immunomodulatory molecules that made their way to the immune system, quelling inflammation. But, in mice that had the NOD2 and ATG16L1 genes, the molecules failed to make their way into the immune system, highlighting the means by which the defective genes can cause bowel problems.
The researchers also report that isolated human immune cells with the defective genes also did not respond to the same type of OMVs. But, the team also found that if the molecules were fed directly to the mice, rather than relying on a messenger for transport, they did wind up in the immune system suggesting that the NOD2 and ATG16L1 genes cause problems with the transport mechanism not the molecules or the communication between them and immune system.
The finding by the team also indicates that it might be possible to create a therapy of molecules that human patients with bowel problems can ingest—one that will offer the same inflammation suppression benefits as those without the genetic defects, by bypassing the transport issues.
More information: H. Chu et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease, Science (2016). DOI: 10.1126/science.aad9948
Abstract
Inflammatory bowel disease (IBD) is associated with risk variants in the human genome and dysbiosis of the gut microbiome, though unifying principles for these findings remain largely undescribed. The human commensal Bacteroides fragilis delivers immunomodulatory molecules to immune cells via secretion of outer membrane vesicles (OMVs). We reveal that OMVs require IBD-associated genes, ATG16L1 and NOD2, to activate a non-canonical autophagy pathway during protection from colitis. ATG16L1-deficient dendritic cells do not induce regulatory T cells (Treg) to suppress mucosal inflammation. Immune cells from human subjects with a major risk variant in ATG16L1 are defective in Treg responses to OMVs. We propose that polymorphisms in susceptibility genes promote disease through defects in 'sensing' protective signals from the microbiome, defining a potentially critical gene-environment etiology for IBD.
Journal information: Science
© 2016 Medical Xpress