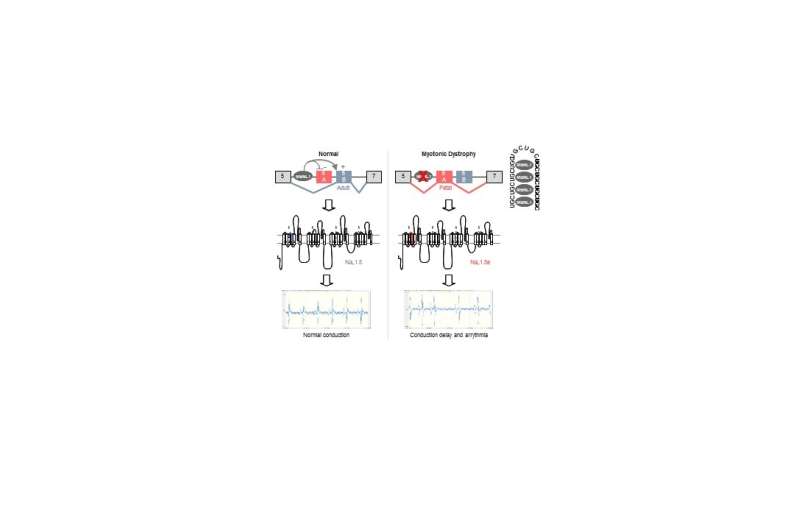
MBNL proteins regulate the switch from exon 6A in embryonic heart to exon 6B in adult. In myotonic dystrophic patients, titration of MBNL protein by RNA containing expanded CUG repeats leads to expression of a fetal splicing form of the cardiac sodium channel, SCN5A, inappropriate to adult heart physiology, ultimately resulting in cardiac conduction delay and heart arrhythmias, which are two key features of myotonic dystrophy. Credit: Nature Communications
An international joint research group found that the cause of heart arrhythmia in myotonic dystrophy was RNA abnormalities in the sodium channel in the heart, clarifying the symptom's mechanism. This finding will be helpful in prevention and early intervention of death in this disease, leading to the development of new treatment.
Myotonic dystrophy, or DM is the most common muscular dystrophy in adults. However, there are currently no cure for DM and there are significant cases of sudden death suspected to be due to heart arrhythmia.
RNA abnormalities have drawn attention as the cause of various systemic manifestations of DM. That is, abnormally expanded RNA accumulates in the nuclei and sequesters proteins necessary for RNA splicing. Therefore, it is thought that improperly controlled splicing of various mRNA result in abnormalities of many proteins, manifesting various conditions. For example, in 2002, Masanori Takahashi, currently Professor at Graduate School of Medicine Osaka University and colleagues suggested that muscle stiffness, a cardinal feature of DM, was caused by decrease in chloride channel proteins due to mis-splicing, but the cause of heart arrhythmia has been unknown for a long time.
A research group led by Professor Takahashi in collaboration with researchers in the France, United States, Germany, Finland, Spain and Taiwan found that there were RNA splicing abnormalities in the cardiac sodium channel in DM patients. The group electrophysiologically examined the function of an abnormal sodium channel and found that it was compromised. From these findings, it was suggested that patients manifested conditions similar to a genetic disease called Brugada syndrome, which led to fatal heart arrhythmia.
When this group introduced RNA abnormalities manifested in patients in mice, heart arrhythmia was observed. In addition, a computer simulation demonstrated that the compromised sodium channel function caused abnormalities in an electrocardiogram, which was similar to abnormalities in patients.
From these findings, it was concluded that heart arrhythmia and sudden death in DM patients were caused by RNA splicing abnormalities in the sodium channel.
Repeat disorders include DM and other neurodegenerative disorders. Therefore, this group's research method and the idea of pathological mechanisms involving RNA abnormalities will be applied to pathological research on various diseases.
More information: Fernande Freyermuth et al, Splicing misregulation of SCN5A contributes to cardiac-conduction delay and heart arrhythmia in myotonic dystrophy, Nature Communications (2016). DOI: 10.1038/ncomms11067
Journal information: Nature Communications
Provided by Osaka University