(HealthDay)—Guidelines have been developed for neoadjuvant chemotherapy use for newly diagnosed, advanced ovarian cancer. The clinical practice guideline was published online Aug. 8 in the Journal of Clinical Oncology.
Alexi A. Wright, M.D., M.P.H., from Harvard Medical School in Boston, and colleagues offer guidance to clinicians regarding the use of neoadjuvant chemotherapy and interval cytoreduction in women with stage IIIC or IV epithelial ovarian cancer. The primary evidence base for recommendations was four phase III clinical trials.
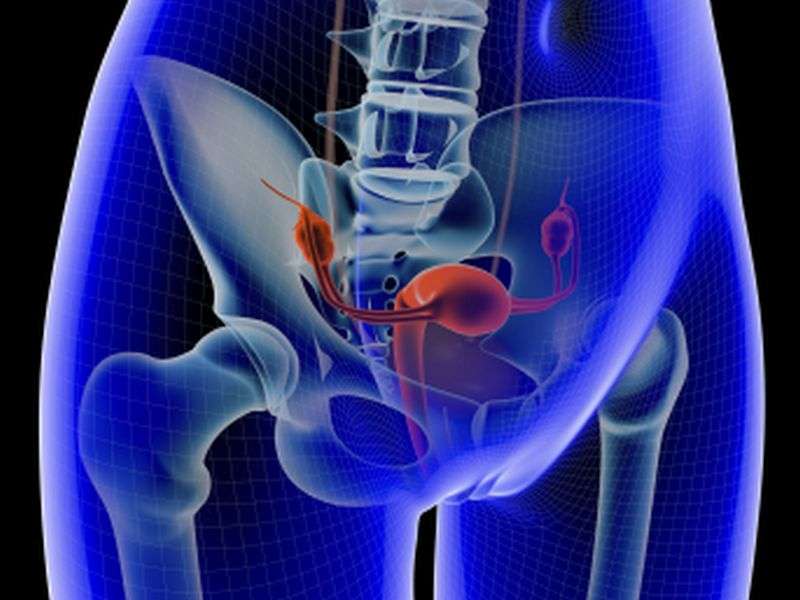
The authors note that all women with suspected stage IIIC or IV invasive epithelial ovarian cancer should undergo gynecologic oncologist assessment before initiation of treatment. The primary clinical evaluation should include abdomen and pelvis computed tomography (CT) and chest imaging (ideally CT). Neoadjuvant chemotherapy is recommended for women with a high perioperative risk profile or a low likelihood of achieving cytoreduction to <1 cm of residual disease. Women with potentially resectable disease who are fit for primary cytoreductive surgery may receive neoadjuvant chemotherapy or primary cytoreductive surgery. If there is a high likelihood of achieving cytoreduction to <1 cm with acceptable morbidity, primary cytoreductive surgery is preferred. All patients should have confirmation of an invasive ovarian, fallopian tube, or peritoneal cancer before neoadjuvant chemotherapy is delivered.
"For women with advanced ovarian cancer, decision-making regarding first-line treatment should be a process that is shared between clinicians and their patients," the authors write.
Several authors disclosed financial ties to the pharmaceutical industry.
More information:
Abstract
Full Text
Journal information: Journal of Clinical Oncology
Copyright © 2016 HealthDay. All rights reserved.