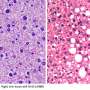
(HealthDay)—Human pregnancy hormone chorionic gonadotropin (hCG) triggers immune suppression by attenuating the processing and release of Caspase-3-dependent interleukin (IL)-16, according to a study published online Jan. 21 in Hepatology.
Noting that pregnancy induces a state of immune tolerance, which can result in spontaneous improvement of clinical symptoms in certain autoimmune diseases, Caroline Steinmetz, from the Johannes Gutenberg University in Mainz, Germany, and colleagues examined the immune suppressive mechanisms of hCG in the liver.
The researchers found that hCG signaling activates SIRT1, which deacetylates FOXO3a, triggering a reduction in expression of pro-apoptotic genes. As a consequence of the absence of Caspase-3 activity, hepatocellular IL-16 was no longer processed and released. There was a reduction in serum levels of IL-16, which is a chemotactic factor for CD4+ lymphocytes, and prevention of migration to injured hepatocytes. Sera from patients with autoimmune hepatitis, hepatitis B virus, hepatitis C virus, and nonalcoholic steatohepatitis had elevated IL-16 levels.
"Here we report that hCG regulates the SIRT1/FOXO3a axis in hepatocytes resulting in immune suppression by attenuating Caspase-3-dependent IL-16 processing and release, which concomitantly prevents auto-aggressive T-cell infiltration to the liver," the authors write.
More information: Full Text (subscription or payment may be required)
Journal information: Hepatology
Copyright © 2017 HealthDay. All rights reserved.