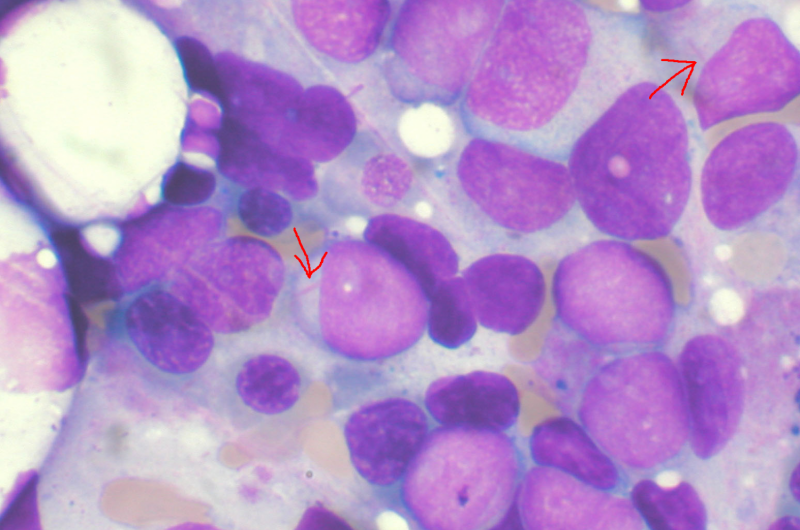
Bone marrow aspirate showing acute myeloid leukemia. Several blasts have Auer rods. Credit: Wikipedia
(HealthDay)—Rydapt (midostaurin) has been approved by the U.S. Food and Drug Administration, in combination with chemotherapy, to treat adults with acute myeloid leukemia (AML) who have a specific genetic mutation dubbed FLT3.
AML, a rapidly spreading cancer that forms in the blood marrow and spikes white blood cells, is projected to be diagnosed in just under 20,000 people, and more than 10,000 are expected to die of the disease annually, the FDA said.
Rydapt is among a class of drugs called kinase inhibitors that are designed to block enzymes that foster cancer cell growth. It was evaluated in a clinical study of more than 700 people who hadn't been treated previously for AML. Common side effects included low white cell count, fever, nausea, headache and muscular/bone pain. A more serious side effect could include lung damage.
Women who are pregnant or nursing shouldn't take Rydapt, which could harm a developing fetus or newborn, the FDA said.
A companion diagnostic test was simultaneously approved to detect the FLT3 gene mutation.
Approval of Rydapt was granted to the Swiss drugmaker Novartis Pharmaceuticals.
More information: Learn more from the FDA.
Copyright © 2017 HealthDay. All rights reserved.