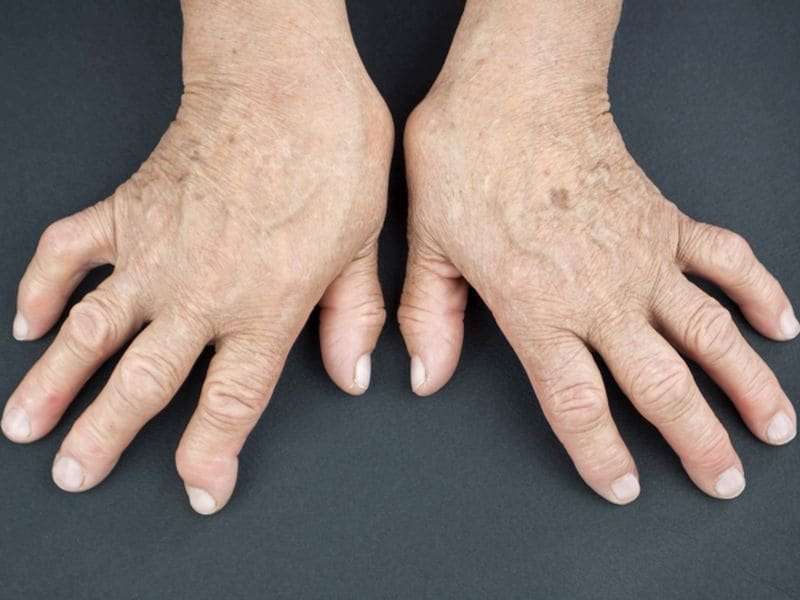
(HealthDay)—Once low rheumatoid arthritis (RA) disease activity is achieved with tocilizumab (TCZ) plus methotrexate (MTX), patients can discontinue MTX without significant disease worsening, according to a study published in the August issue of Arthritis & Rheumatology.
Joel M. Kremer, M.D., from Albany Medical College in New York, and colleagues randomized patients with RA who achieved low disease activity with TCZ plus MTX to either TCZ monotherapy (147 participants) or continuation of TCZ plus MTX (147 participants). Their objective was to assess whether TCZ monotherapy was non-inferior for maintaining clinical responses from week 24 to week 40.
The researchers found that the mean changes in the Disease Activity Score in 28 joints using the erythrocyte sedimentation rate of ≤3.2 from week 24 to week 40 were 0.46 and 0.14 in the TCZ monotherapy arm and the TCZ plus MTX group, respectively (weighted difference between the groups, 0.318). Discontinuation of MTX in TCZ responders was non-inferior to continuation of MTX. Safety events were broadly similar between the groups. Infection was the most common serious adverse event; it occurred in 2.1 percent of patients in the TCZ monotherapy group and 2.2 percent of patients in the TCZ plus MTX group.
"Patients with RA receiving TCZ plus MTX who achieve low disease activity can discontinue MTX without significant worsening of disease activity during the 16 weeks following MTX discontinuation," the authors write.
Several authors disclosed financial ties to pharmaceutical companies, including Genentech, which manufactures tocilizumab and supported the study.
More information: Abstract/Full Text
Copyright © 2018 HealthDay. All rights reserved.