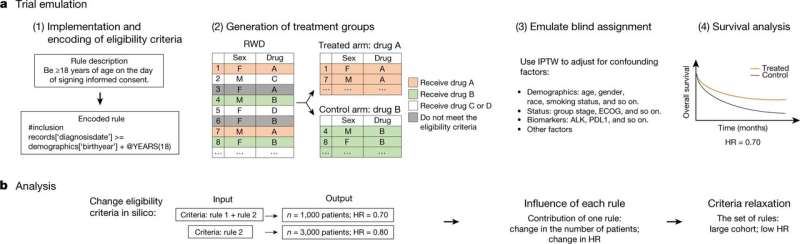
Trial Pathfinder workflow and applications. Credit: Nature (2021). DOI: 10.1038/s41586-021-03430-5
A team of researchers from Stanford University working with biotechnology corporation Genentech, has developed an artificial-intelligence based system that can safely add clinical trial participants that may have previously been excluded. They've published their findings in Nature; Chunhua Weng and James Rogers from Columbia University have published a News & Views piece on the work done by the team in the same journal issue.
In most countries, drugs must pass clinical trials before they are approved for patients to show that, in addition to providing the intended therapy, they are safe. But as the researchers with this new effort note, clinical trials in most places, including the U.S., suffer from one serious drawback—the people that are administered drugs in the clinical trials are specially selected. Most clinical trials, for example, do not allow pregnant women. And most have age requirements. Also, most do no allow those with conditions other than those that are being tested. This filtering process reduces the available pool of possible volunteers, and also unnecessarily excludes many people who may benefit from the therapy. The researchers with this new effort have sought to overcome this problem by building an AI-based system that can safely include more people in clinical trials.
The new system, called Trial Pathfinder, is an AI-based computer system that compares survival outcomes of clinical trial participants included in a large database. As the system analyzes the data, it learns more about which patients are more or less likely to experience problems in a clinical trial for a new drug, based on various factors, such as age, weight, whether they are pregnant and their medical history. The system can then be used to emulate a clinical trial with inclusion of people who would previously have been filtered out. The researchers can then use the information from the system when setting the criteria for their real-world clinical trial. Testing using real-world data on specific applications such as certain types of cancers showed it capable of increasing allowable populations of volunteers in such drug trials to increase by approximately 53%.
More information: Ruishan Liu et al. Evaluating eligibility criteria of oncology trials using real-world data and AI, Nature (2021). DOI: 10.1038/s41586-021-03430-5
Journal information: Nature
© 2021 Science X Network