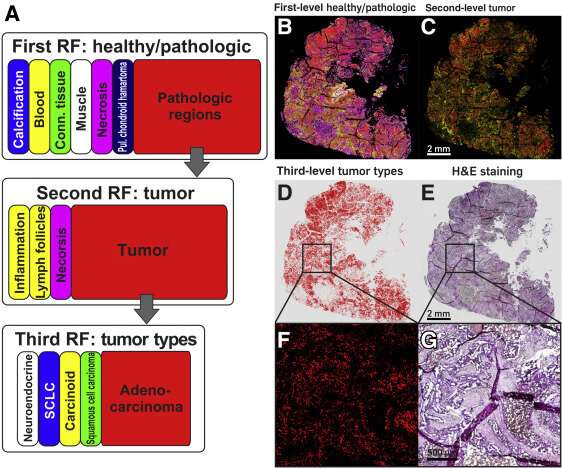
A: Schematic representation of random forest (RF) classifier structure and color code. Top row: The first-level RF differentiates between healthy and pathologic tissue. Middle and bottom rows: The second-level RF (middle row) subdivides the pathologic region into inflammatory infiltrates, lymph follicles, and the tumor region, which is used in the third-level RF (bottom row) to determine the tumor type. Right side: Quantum cascade laser–based infrared imaging of the thin section of a whole slice lung adenocarcinoma tissue. B: Index color image of the result of the first RF classifier, which identifies pathologic regions. C: Subdivision of the pathologic regions using the second RF classifier to identify the tumor. D: Analysis of the tumor region by the third RF classifier to determine the tumor type. E: Hematoxylin and eosin (H&E)–stained image of a thin section of adenocarcinoma tissue. The tumor lesion is identifiable because of the purple hematoxylin staining. F and G: Enlarged sections of boxed areas in D and E show that the red pixels of the index color image (tumor region) match precisely with tumor lesions of the H&E-stained image. Scale bars: 2 mm (B–E); 500 μm (F and G). SCLC, small-cell lung carcinoma.
Lung tumors are divided into various types, such as small cell lung cancer, adenocarcinoma and squamous cell carcinoma. Many rare tumor types and sub-types also exist. This diversity hampers reliable rapid diagnostic methods in everyday clinical practice. In addition to histological typing, the tumor samples also need to be comprehensively examined for certain changes at a DNA level. "Detecting one of these mutations is important key information that influences both the prognosis and further therapeutic decisions," says co-author Professor Reinhard Büttner, head of the Institute of General Pathology and Pathological Anatomy at University Hospital Cologne.
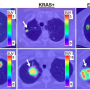
Patients with lung cancer clearly benefit when the driver mutations have previously been characterized: for instance, tumors with activating mutations in the EGFR (epidermal growth factor) gene often respond well to tyrosine kinase inhibitors, whereas non-EGFR-mutated tumors or tumors with other mutations, such as KRAS, do not respond at all to this medication. The differential diagnosis of lung cancer previously took place with immunohistochemical staining of tissue samples and a subsequent extensive genetic analysis to determine the mutation.
Fast and reliable measuring technique
The potential of infrared imaging, IR imaging for short, as a diagnostic tool to classify tissue, called label-free digital pathology, was already shown by the group led by Klaus Gerwert in previous studies. The procedure identifies cancerous tissue without prior staining or other markings and functions automatically with the aid of artificial intelligence (AI). In contrast to the methods used to determine tumor shape and mutations in tumor tissue in everyday clinical practice, which can sometimes take several days, the new procedure only takes around half an hour. In these 30 minutes, it is not only possible to ascertain whether the tissue sample contains tumor cells, but also what type of tumor it is and whether it contains a certain mutation.
Infrared spectroscopy makes genetic mutations visible
The Bochum researchers were able to verify the procedure on samples from over 200 lung cancer patients in their work. When identifying mutations, they concentrated on by far the most common lung tumor, adenocarcinoma, which accounts for over 50 percent of tumors. Its most common genetic mutations can be determined with a sensitivity and specificity of 95 percent compared to laborious genetic analysis. "For the first time, we were able to identify spectral markers that allow for a spatially resolved distinction between various molecular conditions in lung tumors," explains Nina Goertzen from PRODI. A single infrared spectroscopic measurement offers information about the sample which would otherwise require several time-consuming procedures.
A further step towards personalized medicine
The results once again confirm the potential of label-free digital pathology for clinical use. "To further increase reliability and promote a translation of the method as a new diagnostic tool, studies with larger patient numbers adapted to clinical needs and external testing in everyday clinical practice are required," says Dr. Frederik Großerüschkamp, IR imaging project manager. "In order to translate IR imaging into everyday clinical practice, it is crucial to shorten the measuring time, ensure simple and reliable operation of the measuring instruments, and provide answers to questions that are important and helpful both clinically and for the patients."
More information: Nina Goertzen et al, Quantum Cascade Laser-Based Infrared Imaging as a Label-Free and Automated Approach to Determine Mutations in Lung Adenocarcinoma, The American Journal of Pathology (2021). DOI: 10.1016/j.ajpath.2021.04.013
Journal information: American Journal of Pathology
Provided by Ruhr-Universitaet-Bochum