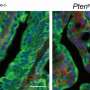
Fig. 1 In vitro and in vivo implementation of the mirReporter assay.(A) Schematic overview of the experimental design (Dox, doxycycline; GFP, green fluorescent protein; IRES, internal ribosome entry site; LTR, long terminal repeat; Neo, neomycin; rtTA3, reverse tetracycline-controlled transactivator 3; PDX, patient-derived xenograft; TRE2, Tet response element 2; UBC, ubiquitin C promoter; 5′cap = ●—). (B) Representative confocal microscopy images of induced GFP (green) expression in HeLa-mirRep145 cells treated with Dox (1 μg/ml, 48 hours) (bottom) compared to phosphate-buffered saline (PBS)–treated control cells (Dox−) (top) (40× objective; scale bar, 50 μm). (C) Representative confocal microscopy images showing decreased GFP expression (green) in Dox-treated HeLa-mirRep145 cells overexpressing miR-145 compared to HeLa-mirRep145 cells overexpressing mock miRNA and let7a [middle: red fluorescent protein (RFP) expression (red); right: GFP expression (green); 40× objective; scale bar, 50 μm]. (D) Fluorescence-activated cell sorting (FACS) based on GFP expression in mirRep145-bearing EwS1-PDX (top) and EwS2-PDX (bottom) cells. (E) Quantitative real-time polymerase chain reaction (qRT-PCR) assessment of mature miR-145 expression in GFP+ and GFP− subpopulations of EwS1-PDX carrying mirRep145 after in vivo induction, tumor dissociation, and cell sorting. (Mean ± SD values of three technical replicates are shown. Statistical analysis was done by unpaired t test; ***P ≤ 0.001.) (F) Immunohistochemical assessment of GFP expression in tumor tissues of a EwS1-PDX-mirRep145–injected control mouse (Dox−) and a Dox-treated mouse (Dox+) (scale bars, 40 μm; inset scale bar, 20 μm).
An aggressive type of cancer has provided a team of researchers supported by the Swiss National Science Foundation with an answer to the question of which tumor cells are at risk of spreading.
Cancer treatment is sometimes complicated by the heterogeneity of the cells that form the tumor mass. The problem is how to identify the few cells that are capable of triggering metastases. Thanks to work carried out by a team of researchers supported by the Swiss National Science Foundation (SNSF), we now have a better understanding of how metastases form and which cells to target in therapy.
The researchers have succeeded in identifying and characterizing the most dangerous cells in Ewing's sarcoma, a very aggressive bone cancer with high potential for spread that primarily affects children and young adults. In particular, among the very active genes of these cells the team pinpointed a gene known to be associated with a poor prognosis. More specifically, this gene promotes the diffusion of cancer cells and the formation of metastases. The work has been published in the journal Science Advances.
The finding constitutes a first step towards the development of more targeted treatments, says Ivan Stamenkovic, professor of experimental pathology at the University Hospital Lausanne (CHUV) and co-author of the paper along with assistant professor Nicolo Riggi, also at CHUV. "Identifying the gene associated with the risk of metastases opens new avenues for research. The protein corresponding to this gene could be used as a potential therapeutic target in eliminating these very aggressive cells," says Stamenkovic.
Telltale glow
To achieve this result, the scientists first had to isolate the cells that form metastases. They took tumors from patients and grew them in conditions that mimic those of the human body to create organoids, i.e. tumor models. The researchers were then able to genetically modify the tumor cells by adding a gene that causes them to express a green fluorescent protein. This gene has been modified so that it can be suppressed by a very small RNA molecule (called microRNA) produced by the cells themselves. Because the cells that form metastases produce very little of this microRNA, they continue to express fluorescent protein. As a result, they appear bright and can be identified by their fluorescent color. "It's a tool that could be used in other types of tumors to understand the nature of aggressive cells," says Riggi.
Cancer cells are more tolerant
Ivan Stamenkovic's research project focuses on the mechanisms responsible for the formation and development of cancers. "In most cancers," says Stamenkovic, "the cells that spread are those that have retained certain properties of stem cells, unlike most of the cells that constitute the tumor mass. These stem-like cells, which are called upon to regenerate tissues, must be able to retain a high degree of flexibility. As a result, they are more tolerant of events such as genetic mutations, and thus more likely to transform into cancerous cells because their defenses are partially or totally suppressed."
More information: Tugba Keskin et al, A live single-cell reporter assay links intratumor heterogeneity to metastatic proclivity in Ewing sarcoma, Science Advances (2021). DOI: 10.1126/sciadv.abf9394
Journal information: Science Advances
Provided by Swiss National Science Foundation
![Fig. 1 In vitro and in vivo implementation of the mirReporter assay.(A) Schematic overview of the experimental design (Dox, doxycycline; GFP, green fluorescent protein; IRES, internal ribosome entry site; LTR, long terminal repeat; Neo, neomycin; rtTA3, reverse tetracycline-controlled transactivator 3; PDX, patient-derived xenograft; TRE2, Tet response element 2; UBC, ubiquitin C promoter; 5′cap = ●—). (B) Representative confocal microscopy images of induced GFP (green) expression in HeLa-mirRep145 cells treated with Dox (1 μg/ml, 48 hours) (bottom) compared to phosphate-buffered saline (PBS)–treated control cells (Dox−) (top) (40× objective; scale bar, 50 μm). (C) Representative confocal microscopy images showing decreased GFP expression (green) in Dox-treated HeLa-mirRep145 cells overexpressing miR-145 compared to HeLa-mirRep145 cells overexpressing mock miRNA and let7a [middle: red fluorescent protein (RFP) expression (red); right: GFP expression (green); 40× objective; scale bar, 50 μm]. (D) Fluorescence-activated cell sorting (FACS) based on GFP expression in mirRep145-bearing EwS1-PDX (top) and EwS2-PDX (bottom) cells. (E) Quantitative real-time polymerase chain reaction (qRT-PCR) assessment of mature miR-145 expression in GFP+ and GFP− subpopulations of EwS1-PDX carrying mirRep145 after in vivo induction, tumor dissociation, and cell sorting. (Mean ± SD values of three technical replicates are shown. Statistical analysis was done by unpaired t test; ***P ≤ 0.001.) (F) Immunohistochemical assessment of GFP expression in tumor tissues of a EwS1-PDX-mirRep145–injected control mouse (Dox−) and a Dox-treated mouse (Dox+) (scale bars, 40 μm; inset scale bar, 20 μm). Cancerous tumors: How likely are they to metastasize?](https://scx1.b-cdn.net/csz/news/800a/2021/cancerous-tumors-how-l.jpg)