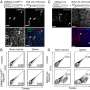
Convalescent plasma induced robust anti-SARS-CoV-2 RBDWT antibody isotypes levels that inhibit ACE2 from binding RBDWT. Convalescent (n = 41) (blue) and uninfected control (n = 26) (gray) plasma (final dilution 1:100) was assessed for IgM (a), IgG (b) and IgA (c) antibody binding to SARS-CoV-2 RBDWT via multiplex. A positive threshold (gray dotted line) was defined as the 75th percentile of antibody binding (MFI) for uninfected control plasma. Statistical analyses were determined using the Mann–Whitney U-test. (d) RBDWT-ACE2 binding inhibition (%) of convalescent (blue) and uninfected control (gray) plasma (diluted 1:100). A positive threshold (gray dotted line) was defined as > 20% ACE2 binding inhibition. (e) A pie chart outlining the percentage of subjects seropositive for anti-RBDWT antibody isotypes [IgM−IgG+IgA+ (green), IgM+IgG+IgA+ (blue), IgM+IgG+IgA− (yellow)] in the inner ring and the percentage of each seropositive subset with ACE2 binding inhibition in the outer red ring. Partial least squares regression (PLSR) were conducted to determine the multivariate relationship between anti-RBD-specific isotype antibodies (IgG, IgA and IgM) and % RBDWT-ACE2 binding inhibition (the % ACE2 binding inhibition illustrated as a color gradient legend on the right yellow—strongest to dark blue—weakest). PLSR Scores (f) and the loadings plot (g). The percentage of variance for each latent variable (LV) is shown in parentheses. Credit: Clinical & Translational Immunology (2022). DOI: 10.1002/cti2.1424
New research from the Peter Doherty Institute of Infection and Immunity (Doherty Institute), The University of Melbourne and WEHI has unlocked key information in our understanding of molecular immune responses to COVID-19.
Following COVID-19 infection, virus-specific antibodies are generated, which can both neutralize the virus and clear the infection. While much has been said about the importance of Immunoglobulin G (IgG) antibodies for protection and control of SARS-CoV-2, the study of the role of Immunoglobin A (IgA) antibodies against SARS-CoV-2 has been relatively neglected throughout the pandemic. Until now.
In a paper published Oct. 23 in Clinical & Translational Immunology, the scientists conducted an experimental study to compare antibody responses to the virus in blood serum from people who had recovered from COVID-19.
University of Melbourne's Samantha Davis, Ph.D. Researcher at the Doherty Institute, is the lead author of the paper.
"In simple terms, we've deconstructed blood in our lab to measure its ability to smother the virus and to activate immune cells to kill SARS-CoV-2," Davis explained.
"While we knew that IgG is very important in the antibody response to clear the virus, we discovered that IgA also plays a key role in neutralizing it in most people."
As neutralizing antibodies are an indicator of immune response and protection against viral infections, this discovery is critical in the context of vaccine development.
University of Melbourne's Dr. Amy Chung is a Laboratory Head the Doherty Institute and one of the senior authors of the paper.
"This research opens the door to new approaches for the development of future vaccines against SARS-CoV-2," Dr. Chung said.
"Our findings are particularly important as IgA is the most abundant antibody present in the mucosa of our respiratory tract, which is the main route of virus infection. This means if we're able to specifically make these antibodies at these vulnerable sites, we now know that we can induce a robust immune response to protect against the virus."
More information: Samantha K Davis et al, Heterologous SARS‐CoV ‐2 IgA neutralising antibody responses in convalescent plasma, Clinical & Translational Immunology (2022). DOI: 10.1002/cti2.1424
Provided by Peter Doherty Institute for Infection and Immunity
![Convalescent plasma induced robust anti-SARS-CoV-2 RBDWT antibody isotypes levels that inhibit ACE2 from binding RBDWT. Convalescent (n = 41) (blue) and uninfected control (n = 26) (gray) plasma (final dilution 1:100) was assessed for IgM (a), IgG (b) and IgA (c) antibody binding to SARS-CoV-2 RBDWT via multiplex. A positive threshold (gray dotted line) was defined as the 75th percentile of antibody binding (MFI) for uninfected control plasma. Statistical analyses were determined using the Mann–Whitney U-test. (d) RBDWT-ACE2 binding inhibition (%) of convalescent (blue) and uninfected control (gray) plasma (diluted 1:100). A positive threshold (gray dotted line) was defined as > 20% ACE2 binding inhibition. (e) A pie chart outlining the percentage of subjects seropositive for anti-RBDWT antibody isotypes [IgM−IgG+IgA+ (green), IgM+IgG+IgA+ (blue), IgM+IgG+IgA− (yellow)] in the inner ring and the percentage of each seropositive subset with ACE2 binding inhibition in the outer red ring. Partial least squares regression (PLSR) were conducted to determine the multivariate relationship between anti-RBD-specific isotype antibodies (IgG, IgA and IgM) and % RBDWT-ACE2 binding inhibition (the % ACE2 binding inhibition illustrated as a color gradient legend on the right yellow—strongest to dark blue—weakest). PLSR Scores (f) and the loadings plot (g). The percentage of variance for each latent variable (LV) is shown in parentheses. Credit: Clinical & Translational Immunology (2022). DOI: 10.1002/cti2.1424 Researchers make strides in the fight against COVID-19](https://scx1.b-cdn.net/csz/news/800a/2022/researchers-make-strid-1.jpg)