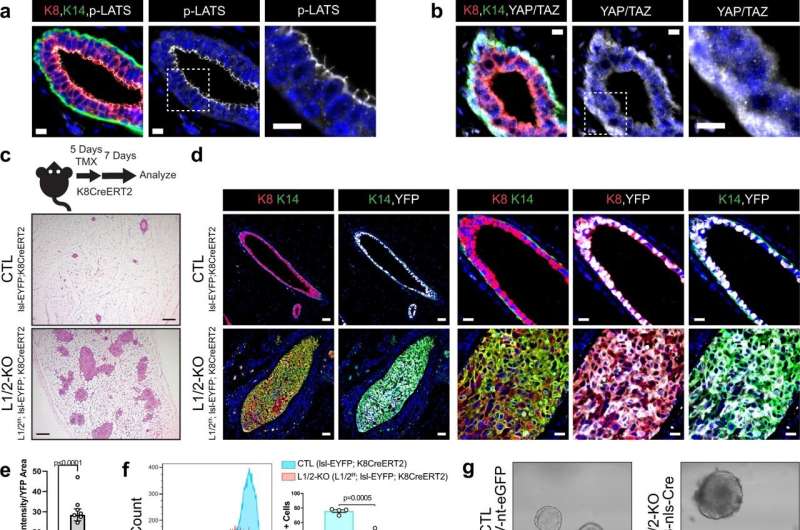
Loss of LATS1/2 in luminal mammary cells induces mammary carcinomas exhibiting luminal-basal plasticity. a, b Immunofluorescence (IF) staining of a phospho-LATS1/2 (Thr1079/1041) or b YAP/TAZ together with K8 and K14 in control mouse mammary ducts (Scale bar, 10 µM). c Diagram of the mouse model used to conditionally delete LATS1/2 in K8+ luminal mammary epithelial cells along with H&E staining of control and Lats1/2f/f;lsl-EYFP;K8CreERT2 mammary glands (Scale bar, 200 µM). d IF staining of K8, K14, and EYFP in control and Lats1/2f/f;lsl-EYFP;K8CreERT2 mammary glands following tamoxifen treatment (Scale bar 50 µM in the left two columns, and 20 µM in the right three columns). e Quantification of K14 intensity/YFP area in mammary ducts of lsl-EYFP;K8CreERT2 (CTL) and Lats1/2f/f;lsl-EYFP;K8CreERT2 (L1/2-KO) mice (n = 5 images from 2 CTL mice, n = 9 images from 2 L1/2-KO mice. Unpaired two-tailed t-test, Data are shown as mean ± SEM). f Flow cytometric profiling for Sca1 expression in CTL and L1/2-KO mammary epithelial cells (n = 4 CTL, n = 3 L1/2-KO. Unpaired two-tailed t-test, Data are shown as mean ± SEM). g Morphology of organoids cultured from control and LATS1/2f/f mice infected with AdK8-nls-Cre (Scale bar, 50 µM). h RNA expression of selected luminal and basal/stem-cell markers in control and LATS1/2f/f organoids infected with AdK8-nls-Cre (n = 4. Unpaired two-tailed t-tests. Data are shown as mean ± SEM). i IVIS live imaging of a primary tumor (red arrow) formed in the mammary gland of a LATS1/2f/f; lsl-EYFP mouse approximately 14 months after intraductal injection with AdK8-nls-Cre compared to an uninjected control mouse. j IVIS imaging of EYFP signal from a representative primary tumor (top) and lung metastases (bottom) formed in a LATS1/2f/f; lsl-EYFP mouse approximately 11 months after intraductal injection with AdK8-nls-Cre compared to organs from an uninjected control mouse. k, l IF staining of k K8, K14, and tdTomato or l Vimentin and tdTomato in a primary tumor formed in a LATS1/2f/f; lsl-tdTomato mouse approximately 13 months after intraductal injection with AdK8-nls-Cre (n = 2 tumors) (Scale bars, 500 µM on low magnification images on left, and 200 µM on high magnification images of regions outlined by white dotted lines on the right). Source data are provided as a source data file. Credit: Nature Communications (2022). DOI: 10.1038/s41467-022-34864-8
Basal-like breast cancers, also known as triple-negative cancers, are an aggressive breast cancer subtype with poor treatment options. The cells of origin for luminal (cells that line the surface of the breast duct) and basal subtypes of breast cancer are not fully understood, but studies suggest that basal-like cancers can arise from luminal epithelial cells.
In a new study, researchers from Boston University Chobanian & Avedisian School of Medicine demonstrated that proper control of a cellular pathway known as the Hippo pathway prevents the development of triple negative breast cancer.
"We found that when this pathway is dysregulated or impaired, luminal epithelial cells in the mammary gland rapidly transition to a basal-like state and develop into triple negative carcinomas," explained corresponding author Bob Varelas, Ph.D., associate professor of biochemistry.
In an experimental model, the researchers conditionally deleted the Lats1 and Lats2 genes, two components of the Hippo pathway, in the luminal epithelium of the mammary glands. When these genes were deleted, the models rapidly develop basal-like mammary carcinomas resembling human basal-like breast cancers. They found that the development of these carcinomas depended on the activity of the Hippo pathway effector proteins YAP and TAZ, and that deletion of these two proteins reversed carcinoma development in their model.
According to the researchers, the gene-expression signature identified from Lats1/2-deleted tumors could be used to identify aggressive features associated with triple negative breast cancers. Since basal-like breast cancers are notoriously difficult to treat, Varelas hopes these findings may be leveraged to help guide new directions for better treatment and improved patient outcomes. "A better understanding of the cellular mechanisms leading to the development of these cancers is essential to identifying new therapeutic options," he said.
These findings appear online in the journal Nature Communications.
More information: Joseph G. Kern et al, Inactivation of LATS1/2 drives luminal-basal plasticity to initiate basal-like mammary carcinomas, Nature Communications (2022). DOI: 10.1038/s41467-022-34864-8
Journal information: Nature Communications
Provided by Boston University School of Medicine