For patients newly diagnosed with Parkinson disease, deferiprone is associated with worse scores in measures of parkinsonism, according to a study published in the Dec. 1 issue of the New England Journal of Medicine.
David Devos, M.D., Ph.D., from the University of Lille in France, and colleagues conducted a phase 2 trial involving patients with newly diagnosed Parkinson disease who had never received levodopa. Participants were randomly assigned to receive oral deferiprone (15 mg/kg body weight twice daily) or matched placebo for 36 weeks in a 1:1 ratio (186 patients in each group). Unless deemed necessary for symptom control, dopaminergic therapy was withheld.
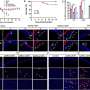
The researchers found that progression of symptoms led to initiation of dopaminergic therapy in 22.0 and 2.7 percent of participants in the deferiprone and placebo groups, respectively. The mean Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale score at baseline was 34.3 in the deferiprone group and 33.2 in the placebo group and increased by 15.6 and 6.3 points, respectively.
There was a greater decrease observed in nigrostriatal iron content in the deferiprone versus placebo group. The main serious adverse events with deferiprone were agranulocytosis and neutropenia in two and three participants, respectively.
"Deferiprone was not associated with benefit as compared with placebo in measures of the progression of Parkinson's disease, and there was evidence of clinical worsening," the authors write. "Deferiprone was associated with adverse events."
ApoPharma and Chiesi provided deferiprone and placebo for the study.
More information: David Devos et al, Trial of Deferiprone in Parkinson's Disease, New England Journal of Medicine (2022). DOI: 10.1056/NEJMoa2209254
Douglas Galasko et al, Lack of Benefit of Iron Chelation in Early Parkinson's Disease, New England Journal of Medicine (2022). DOI: 10.1056/NEJMe2213120
Journal information: New England Journal of Medicine
Copyright © 2022 HealthDay. All rights reserved.