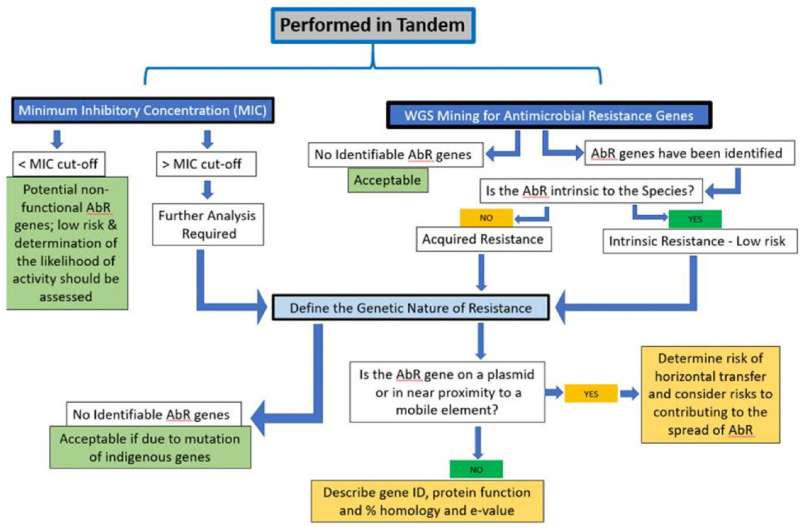
Phenotypic and Genotypic analysis of antimicrobial resistance genes for consideration of strain safety. Credit: Regulatory Toxicology and Pharmacology (2022). DOI: 10.1016/j.yrtph.2022.105266
In past decades, the safety of many probiotics available to consumers was anchored in knowledge of their long history of safe consumption in humans. However, the range of probiotics is expanding to include non-traditional probiotic strains, which may bring health benefits but are not typically present in food sources and do not have a history of safe use.
A group of industry and government scientists convened under the auspices of the United States Pharmacopeia's Probiotic Expert Panel recently set out to review current approaches to assessing the safety of probiotics from a scientific perspective, while also looking at what regulators currently require.
The paper, newly published in Regulatory Toxicology and Pharmacology, summarizes scientific frameworks for assessing the safety of probiotics used in foods and dietary supplements, including the importance of comprehensive genomic characterization. The paper questions the value of animal testing for probiotics intended for human use. Further, the paper considers emerging technologies for safety testing as well as how manufacturing practices play an important role in the safety of the final product.
Recognizing that regulatory approaches to probiotic safety vary greatly around the world, the paper focuses on scientifically meaningful approaches for safety assessment. Risk tiers for probiotics are identified, based on available information on history of safe use. For strains of species recognized by authoritative bodies as having a sufficient history of safe use, and for which their genomes show no genes of concern, no additional testing is proposed. But if genes of concern are present or a history of safe use is lacking, additional testing is warranted. Safety of genetically modified probiotics or probiotics used as drugs was not addressed in this paper.
Dr. Mary Ellen Sanders, the Executive Science Officer for the International Scientific Association for Probiotics and Prebiotics (ISAPP), and a co-author of the paper, commented, "The current situation, with different countries having different criteria for showing safety of the same probiotic strain, is not ideal. This paper calls for a more harmonized regulatory approach to probiotic safety, based on sound scientific principles."
Sanders says that while probiotics in foods and supplements are used widely and have a good safety record, establishing safety of innovative strains may require more extensive testing prior to marketing.
Amy Roe, Principal Toxicologist, Product Safety & Regulatory Affairs, The Procter & Gamble Company, led the writing effort. She says, "We hope that this review, which includes the use of modern technologies and approaches, will provide helpful recommendations for completing a comprehensive safety assessment of a probiotic."
More information: Amy L. Roe et al, Considerations for determining safety of probiotics: A USP perspective, Regulatory Toxicology and Pharmacology (2022). DOI: 10.1016/j.yrtph.2022.105266
Provided by International Scientific Association for Probiotics and Prebiotics