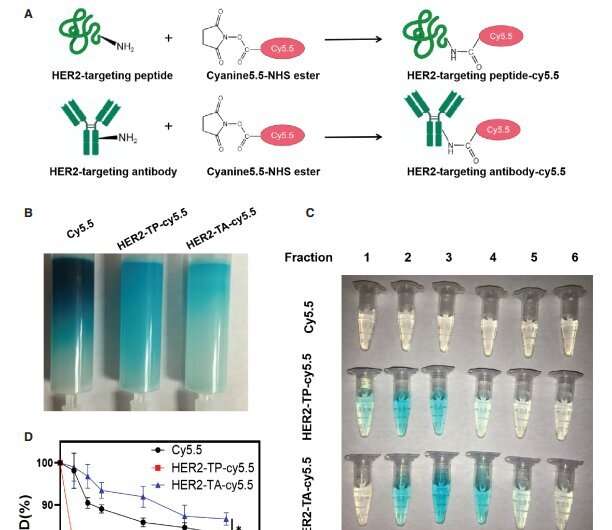
Schematic diagram and pharmacokinetics of HER2-TP-cy5.5 and HER-TA-cy5.5 conjugate synthesis. (A) Schematic representation of the utilization of HER2-targeting peptide/antibody and Cyanine5.5-NHS ester (Cy5.5) to form conjugates. (B) Purification of the conjugates, (C) Collection of the purified components. (D) The circulation time of free Cy5.5 and Cy5.5-labeled peptide/antibody after intravenous injection in mice via the tail vein. mean±SD; n=3; *p < 0.05, ***p < 0.001. HER2-TP-cy5.5: HER2-targeting peptide-cy5.5 conjugate; HER2-TA-cy5.5: HER2-targeting antibody-cy5.5 conjugate. Credit: BIO Integration (2023). DOI: 10.15212/bioi-2023-0006
Antibody-drug conjugates (ADCs) have advantages including target specificity, wide therapeutic index and prolonged circulation half-life. A key limitation of ADCs, however, is the large size (∼150 kDa), which markedly slows diffusion through the interstitium of solid tumors and prevents efficient penetration.
To address the size issue of ADCs in targeted drug delivery, the authors of an article published in BIO Integration developed a HER2-targeting peptide-mertansine conjugate (HER2-TPMC) and conducted a head-to-head comparison with HER2-targeting antibody-mertansine conjugate (HER2-TAMC) as a possible alternative for high-penetration breast cancer therapeutics.
As expected, a pharmacokinetic (PK) assay revealed that HER2-TP had lower levels persisting in the circulation after 1 hour (∼75%) compared to 85% of HER2-targeting antibody (HER2-TA). The cellular cytotoxic effect of HER2-TPMC was similar to HER2-TAMC in the HER2+ BT474 breast cancer cell line, thus demonstrating similar bioactivity of both conjugates. HER2-TPMC not only revealed higher uptake and specificity in in vitro 3D spheroid cultures compared to the parental drug, mertansine, but HER2-TPMC also had a significant retention in the spheroids.
This finding was in stark contrast to HER2-TAMC, a large-sized conjugate which was not able to penetrate the spheroid barrier, thus resulting minimal penetration. In vivo tumoral uptake in a BT474 orthotopic model indicated increased tumor uptake and penetration of HER2-TP compared to parental drug and HER2-TAMC.
The authors successfully developed a HER2-targeting peptide-mertansine conjugate with specific cellular uptake that resulted in longer retention times in vitro and in vivo. HER2-TPMC (∼5 kDa in size) exhibited rapid tissue penetration and enhanced tumoral uptake and retention in vitro and in vivo. Therefore, HER2-TPMC is a reasonable alternative for HER2-positive cancer chemotherapeutics.
More information: Yixia Liang et al, HER2-targeting Peptide Drug Conjugate with Better Penetrability for Effective Breast Cancer Therapy, BIO Integration (2023). DOI: 10.15212/bioi-2023-0006
Provided by Compuscript Ltd