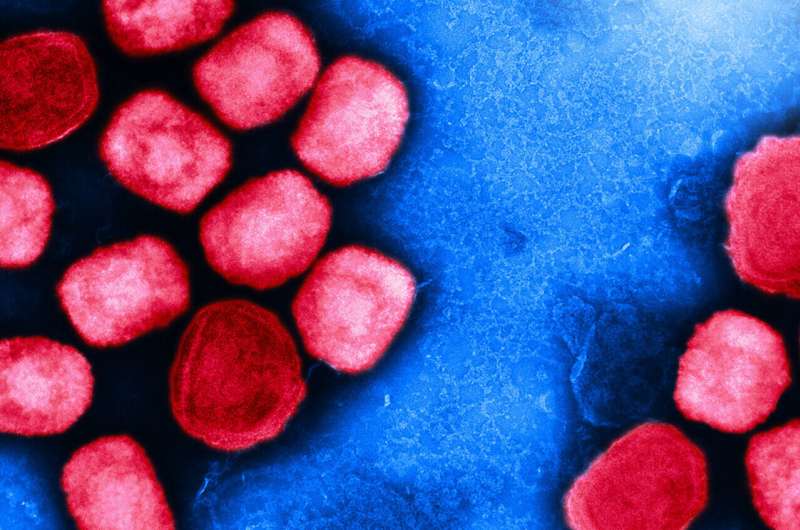
Colorized transmission electron micrograph of mpox virus particles (red) cultivated and purified from cell culture. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Credit: NIAID
A cohort study of persons with confirmed mpox infections who were treated with the novel antiviral tecovirimat has found that the HIV status of the patients did not impact treatment outcomes. The findings are published in Annals of Internal Medicine.
In May 2022, an outbreak of the zoonotic DNA orthopoxvirus mpox began in Europe. The most recent outbreak occurred mostly among men who have sex with men (MSM) and has been associated with significant rates of coincident sexually transmitted infections. Almost half of mpox infections during this outbreak occurred in people with HIV, but it is unknown to what degree HIV acts as an independent risk factor for mpox acquisition.
Researchers from Columbia University Medical Center and New York Presbyterian Hospital conducted a retrospective cohort study of 196 people treated with tecovirimat from 20 June to 29 August 2022. Of the 196 participants, 154 were diagnosed with mpox and 72 were persons with HIV. The authors report that indications for tecovirimat treatment were similar between persons with and without HIV.
They also report that only 22 percent of participants had nonsevere adverse effects, and only 4 participants had serious adverse events. Three of the four participants who experienced serious adverse were persons with HIV.
More information: Tecovirimat Treatment of People With HIV During the 2022 Mpox Outbreak, Annals of Internal Medicine (2023). DOI: 10.7326/M22-3132. www.acpjournals.org/doi/10.7326/M22-3132
Journal information: Annals of Internal Medicine
Provided by American College of Physicians