Once-daily semaglutide 25 mg or 50 mg is superior to 14 mg for glycemic control among adults with type 2 diabetes, according to a study published online June 25 in The Lancet.
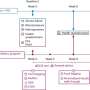
Vanita R. Aroda, M.D., from Brigham and Women's Hospital in Boston, and colleagues conducted a global, multicenter, randomized phase 3b trial at 177 sites in 14 countries involving adults with type 2 diabetes.
Participants had glycated hemoglobin (HbA1c) of 8.0 to 10.5 percent and body mass index of 25.0 kg/m2 or greater and were receiving stable daily doses of one to three oral glucose-lowering drugs. Participants were randomly assigned to 68 weeks of once-daily oral semaglutide 14 mg, 25 mg, or 50 mg (536, 535, and 535 participants, respectively). Change in HbA1c from baseline to week 52 was evaluated as the primary end point.
The researchers found that the mean changes in HbA1c at week 52 were −1.5, −1.8, and −2.0 percentage points with oral semaglutide 14 mg, 25 mg (estimated treatment difference, −0.27), and 50 mg (estimated treatment difference, −0.53), respectively. Adverse events were reported by 76, 79, and 80 percent of participants in the 14-mg, 25-mg, and 50-mg groups, respectively. Gastrointestinal disorders were mostly mild to moderate and occurred more often with oral semaglutide 25 mg and 50 mg versus 14 mg.
"Participants with inadequately controlled type 2 diabetes on a stable daily regimen of one to three oral glucose-lowering drugs at screening showed both superior glycemic control and superior bodyweight reduction with once-daily oral semaglutide 25 mg or 50 mg compared with 14 mg," the authors write.
More information: Vanita R Aroda et al, Efficacy and safety of once-daily oral semaglutide 25 mg and 50 mg compared with 14 mg in adults with type 2 diabetes (PIONEER PLUS): a multicentre, randomised, phase 3b trial, The Lancet (2023). DOI: 10.1016/S0140-6736(23)01127-3
Christina H Sherrill et al, The pursuit of optimal semaglutide dosing in type 2 diabetes continues, The Lancet (2023). DOI: 10.1016/S0140-6736(23)01233-3
Journal information: The Lancet
Copyright © 2023 HealthDay. All rights reserved.