Credit: Pixabay/CC0 Public Domain
Swiss pharmaceutical giant Novartis on Monday reported positive interim Phase III results for a new drug being studied to treat a rare kidney disease.
Novartis said the treatment, Iptacopan, had achieved significant clinical results over nine months in a study, dubbed APPLAUSE-IgAN, for patients with immunoglobulin A (IgA) nephropathy, or IgAN.
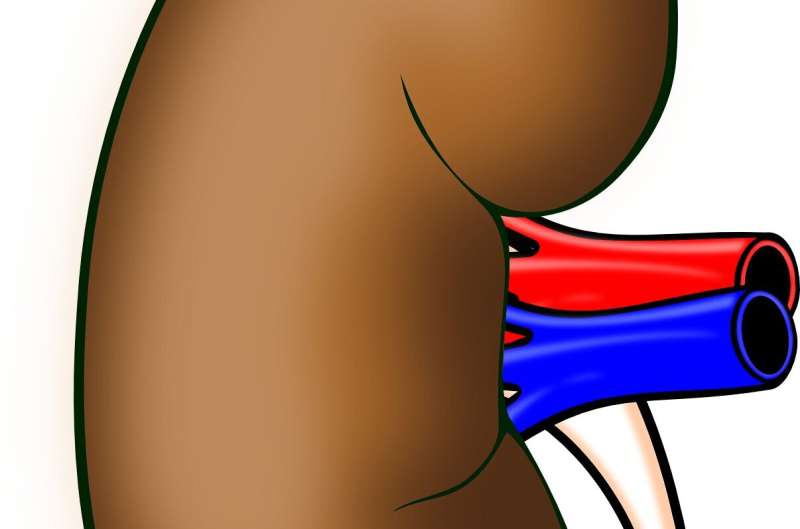
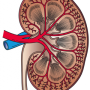
IgAN is a rare autoimmune disease mostly affecting young adults, which can lead to kidney failure.
The study is to continue for a further 15 months. In total, it will cover around 450 adult patients with primary IgAN who are at high risk of progressing to kidney failure.
The company said that Iptacopan had, in the interim results, "demonstrated superiority versus placebo in proteinuria (protein in urine) reduction and provided a clinically meaningful and highly statistically significant proteinuria reduction".
The Swiss group added it intended to submit the results to the US drug agency, the FDA, for accelerated approval in 2024.
About 25 out of every million people worldwide are diagnosed with IgAN annually.
Novartis said IgAN, an autoimmune reaction to an abnormal form of IgA, resulted in the formation of immune complexes which deposit in the kidney, leading to progressive damage and eventual loss of kidney function.
It added up to 30 percent of people who have IgAN with persistent higher levels of proteinuria may suffer kidney failure within 10 years.
"Iptacopan is a pipeline in a tablet," said Stefan Schneider, analyst at Vontobel, in a market commentary.
He said the treatment had already produced positive results for paroxysmal nocturnal hemoglobinuria, a rare disease characterized by sudden, often nocturnal, outbreaks of red blood cell destruction.
Schneider estimated peak sales potential for the new drug at $2.7 billion—$1.7 billion to treat IgA nephropathy alone.
Among rare diseases, IgA nephropathy is the most common form of glomerulonephritis—inflammation of kidney filters.
Novartis is conducting work on Iptacopan for several rare diseases, including paroxysmal nocturnal hemoglobinuria, IgA nephropathy and C3 glomerulopathy.
The Food and Drug Administration (FDA) has granted therapeutic breakthrough status to the treatment.
In early market trading on Monday, Novartis shares were up 0.35 percent to 94.20 Swiss francs ($103) in an overall market adding 0.48 percent.
© 2023 AFP