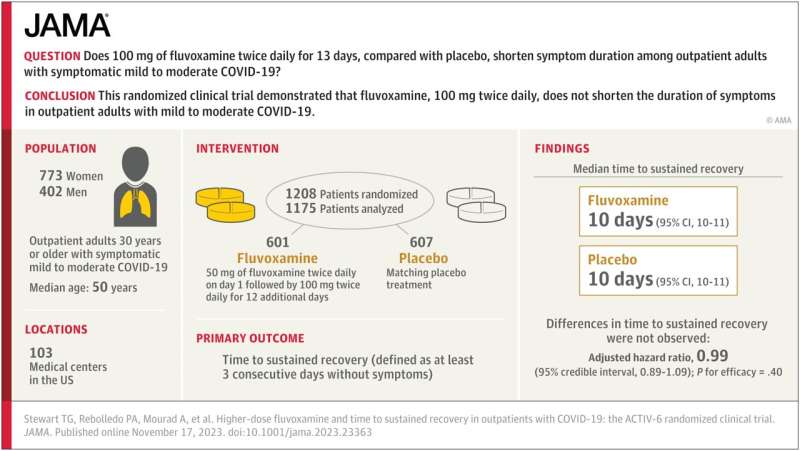
Visual Abstract. Higher-Dose Fluvoxamine and Time to Sustained Recovery in Outpatients With COVID-19. Credit: JAMA (2023). DOI: 10.1001/jama.2023.23363
A new research article from Thomas Stewart, an associate professor of data science at the University of Virginia, examining the COVID-19 treatment fluvoxamine has been published in JAMA.
The study—which Stewart co-authored with physicians Paulina Rebolledo of Emory University and Ahmad Mourad of Duke University and the ACTIV-6 Study Group—examines whether 100 mg of fluvoxamine taken twice daily for 13 days by outpatient adults with mild to moderate cases of COVID-19 can shorten the duration of symptoms.
The researchers analyzed a randomized clinical trial of 1,175 participants in the United States who had COVID-19 while omicron subvariants were circulating. They found that when compared to a placebo group, 100 mg of fluvoxamine taken twice per day did not reduce how long patients with mild to moderate COVID experienced symptoms.
Prior to this study, the impact of a high dosage of fluvoxamine on symptom duration for these types of cases of COVID-19 had been uncertain.
Stewart specializes in biostatistics, clinical trials, and clinical research education. He also serves as director of the Ph.D. program at the School of Data Science.
More information: Thomas G. Stewart et al, Higher-Dose Fluvoxamine and Time to Sustained Recovery in Outpatients With COVID-19, JAMA (2023). DOI: 10.1001/jama.2023.23363
Journal information: Journal of the American Medical Association
Provided by University of Virginia