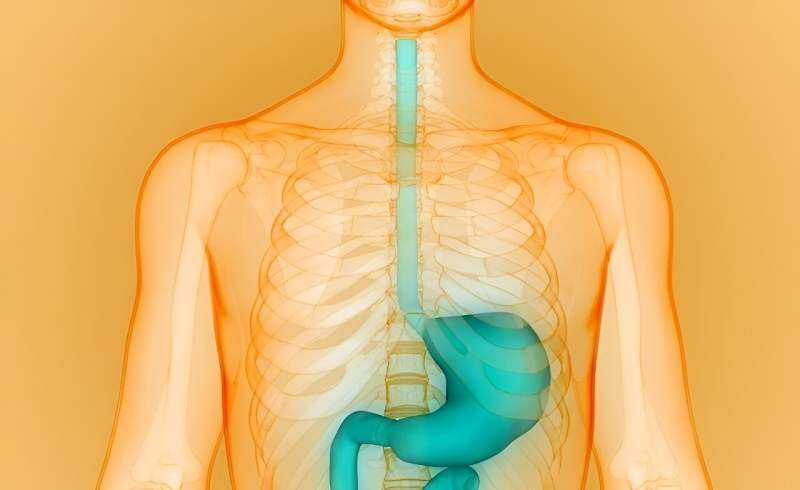
The U.S. Food and Drug Administration has approved Takeda's Eohilia (budesonide oral suspension) as the first and only oral treatment for eosinophilic esophagitis (EoE).
The oral corticosteroid therapy is approved for individuals aged 11 years and older and will be available in 2mg/10mL single-dose stick packs by the end of February. The approval calls for twice-daily use for 12 weeks of treatment.
The approval is based on two multicenter, randomized, double-blind, parallel-group, placebo-controlled 12-week studies in patients (ages 11 to 56 and 11 to 42 years, respectively).
The two studies revealed that significantly more patients receiving Eohilia achieved histologic remission versus those receiving placebo (study 1: 53.1 versus 1 percent; study 2: 38 versus 2.4 percent). From baseline, the absolute change in the Dysphagia Symptom Questionnaire combined score was −10.2 with Eohilia versus −6.5 with placebo in study 1 and −14.5 and −5.9, respectively, in study 2.
Compared with those taking placebo, more patients receiving Eohilia experienced no dysphagia or only experienced dysphagia that "got better or cleared up on its own" during the last two weeks of each study.
"For people living with eosinophilic esophagitis, sitting down for a meal can include painful and difficult swallowing, chest pain, and a choking sensation," Brandon Monk, senior vice president and head of the U.S. Gastroenterology Business Unit at Takeda, said in a statement.
"With Eohilia, patients and their physicians now have the first and only FDA-approved oral treatment option for EoE that was shown during two 12-week clinical studies to reduce esophageal inflammation and improve the ability to swallow."
More information: More Information
Copyright © 2024 HealthDay. All rights reserved.