This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
proofread
Oral interleukin-23-receptor antagonist peptide shows greater efficacy than placebo for plaque psoriasis
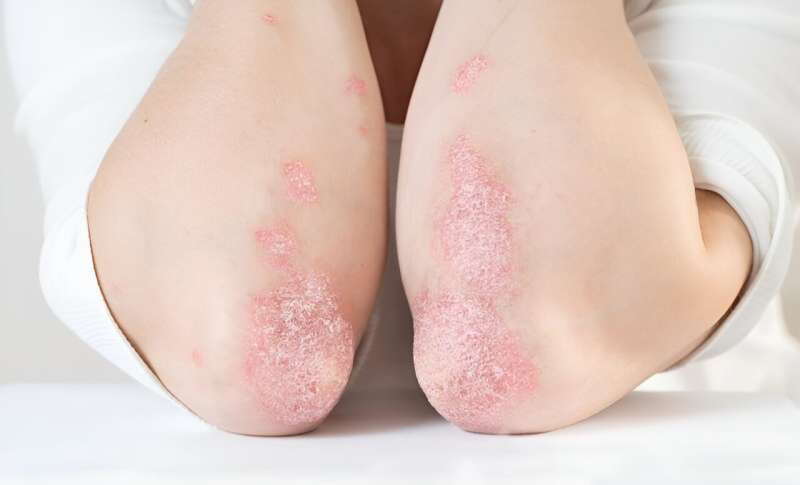
For patients with moderate-to-severe plaque psoriasis, the interleukin-23-receptor antagonist peptide JNJ-77242113 shows greater efficacy than placebo, according to a study published in the Feb. 8 issue of the New England Journal of Medicine.
Robert Bissonnette, M.D., from Innovaderm Research in Montreal, and colleagues conducted a phase 2 dose-finding trial involving 255 patients with moderate-to-severe plaque psoriasis who were randomly assigned to receive JNJ-77242113 at a dose of 25 mg once daily, 25 mg twice daily, 50 mg once daily, 100 mg once daily, or 100 mg twice daily or placebo for 16 weeks.
A reduction from baseline of at least 75 percent in the Psoriasis Area and Severity Index (PASI) score (PASI 75 response) at week 16 was the primary endpoint.
The researchers found that the percentage of patients with a PASI 75 response was higher among those receiving JNJ-77242113 versus placebo (37, 51, 58, 65, and 79 percent in the 25-mg once-daily, 25-mg twice-daily, 50-mg once-daily, 100-mg once-daily, and 100-mg twice-daily groups, respectively, versus 9 percent), with a significant dose-response relationship observed. COVID-19 and nasopharyngitis were the most common adverse events.
The percentages of patients with at least one adverse event was similar in the combined JNJ-77242113 dose group and the placebo group (52 and 51 percent, respectively).
"In this phase 2 trial, JNJ-77242113, an oral interleukin-23-receptor antagonist peptide, showed a dose-response relationship and greater efficacy than placebo," the authors write.
The study was funded by Janssen Research and Development, which is developing JNJ-77242113.
More information: Robert Bissonnette et al, An Oral Interleukin-23–Receptor Antagonist Peptide for Plaque Psoriasis, New England Journal of Medicine (2024). DOI: 10.1056/NEJMoa2308713
Joel M. Gelfand, Psoriasis—More Progress but More Questions, New England Journal of Medicine (2024). DOI: 10.1056/NEJMe2314345
Copyright © 2024 HealthDay. All rights reserved.