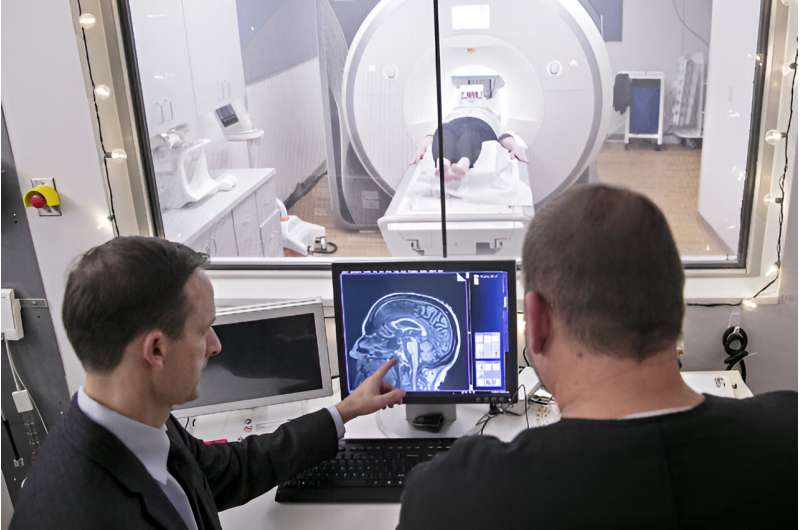
David Vaillancourt, Ph.D., (left) is a senior author on a new study linking abnormal amyloid in the blood with brain changes on diffusion MRI. Credit: University of Florida
New research suggests there is a link between abnormal blood levels of amyloid—a protein associated with Alzheimer's disease—and subtle changes in brain microstructures on a type of MRI, findings that could lead to a new way to detect Alzheimer's earlier in people with no clinical signs.
Researchers analyzed the results of 128 human participants with and without dementia from the 1Florida Alzheimer's Disease Research Center who underwent imaging scans using an established diagnostic tool called positron emission tomography, or PET, which can detect amyloid plaques in the brain, a hallmark of Alzheimer's disease.
Even when a PET scan was negative for amyloid and a participant free of dementia symptoms, researchers found there was an association in those who showed abnormal amyloid levels in the blood and structural abnormalities in the brain detected through a newer method called diffusion MRI, also known as "free-water" imaging.
A team led by investigators from UF's Evelyn F. and William L. McKnight Brain Institute and the Norman Fixel Institute for Neurological Diseases at UF Health reported that the results represent a novel finding that free-water imaging is sensitive to early stages of decline in brain tissue and tiny structures in key parts of the brain—even when a PET scan is negative. The results were published in Alzheimer's & Dementia.
"Previously people would say one of the earliest events you would see is amyloid positivity in the brain on a PET scan," said senior author David Vaillancourt, Ph.D., a professor and chair of the UF College of Health & Human Performance's department of applied physiology and kinesiology. "Our findings suggest there seem to be events occurring both in the blood and in the brain before you detect amyloid positivity in the brain."
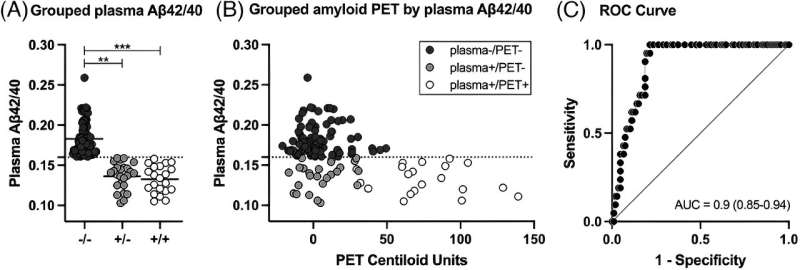
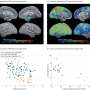
Relationship between plasma amyloid-β (Aβ42/40) and Aβ positron emission tomography (PET). (A) Grouped strip plots representing individual plasma Aβ42/40 measurements and groups means (horizontal black lines) in plasma-/PET- (dark gray: -/-), plasma+/PET- (light gray: +/-), and plasma+/PET+ (white: +/+) groups. Statistical denotations for nonparametric independent samples permutation tests: ** pFDR < = 0.01, *** pFDR < = 0.001. (B) Grouped Aβ PET Centiloid units by plasma Aβ42/40 using the same group color scheme as in plot A. An outlier data point from a single participant in the plasma+/PET+ group was censored for improved visualization but was included in all analyses (PET Centiloid = 230, plasma Aβ42/40 = 0.138). The horizontal dashed lines in plots A and B represent the cut-point for separating plasma Aβ42/40 positive (< 0.16) and negative participants. (C) Receiver operating characteristic (ROC) area-under-the-curve (AUC) showing the correspondence between plasma Aβ42/40 and Aβ PET status (+/-). Credit: Alzheimer's & Dementia (2024). DOI: 10.1002/alz.13693
Blood levels of amyloid were determined using Quest AD-Detect amyloid beta 42/40, a plasma blood test developed by Quest Diagnostics to help assess risk of Alzheimer's pathology. Collaborators from UF, the University of Miami and Mount Sinai Medical Center in Miami Beach then analyzed diffusion MRI results showing the amount of free-water, or fluid unconstrained by brain tissue.
Two main mechanisms affect free-water: atrophy, which occurs when cells are dying, and inflammation, Vaillancourt said. The new study builds upon his lab's discovery and validation of free-water imaging as a reliable, noninvasive biomarker for another neurodegenerative malady, Parkinson's disease.
In the new study, participants who had positive blood tests for amyloid but negative PET scans for amyloid were shown to have brain changes on diffusion MRI, including decreased cortical volume and thickness, increased free-water in 24 outer and inner parts of the brain and decreased tissue microstructure in 66 total regions, as compared to those with a negative amyloid blood test and a negative amyloid PET scan, the researchers reported.
Currently, to assess patients for Alzheimer's disease, physicians use a combination of medical history, neurological exams, cognitive and functional assessments, and additional tests that may include brain imaging, a spinal tap of cerebrospinal fluid and blood tests. Finding new methods and biomarkers to detect the disease earlier and at less expense could open the door to new clinical trials of experimental drugs to slow, prevent or treat the condition and help intervene sooner with currently available medications, Vaillancourt said.
The next step in this line of research to better correlate these findings, he said, is to follow the participants to see if those with positive amyloid blood tests become amyloid-positive on a PET scan as well as how free-water and blood change over time and how well these changes correlate with symptoms and cognitive testing and eventual clinical diagnosis of Alzheimer's disease.
"We want to follow them over time to better understand the trajectory of change," Vaillancourt said.
More information: Jesse C. DeSimone et al, Diffusion MRI relates to plasma Aβ42/40 in PET negative participants without dementia, Alzheimer's & Dementia (2024). DOI: 10.1002/alz.13693
Journal information: Alzheimer's & Dementia
Provided by University of Florida