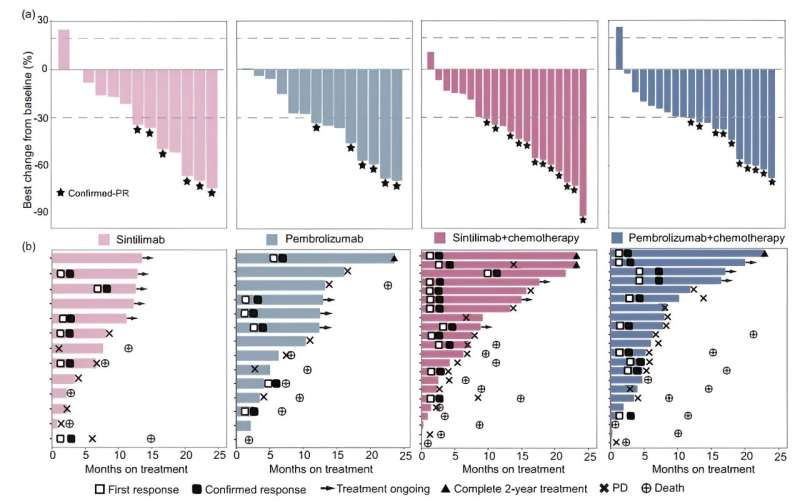
The best change in target lesion size and treatment duration for all patients are illustrated in waterfall and swimmer plots. Credit: Science China Press
A study published in the journal Science Bulletin was led by Yi-Long Wu (Guangdong Lung Cancer Institute, Guangdong Provincial People's Hospital, Chinese Thoracic Oncology Group (CTONG)).
In this open-label, phase 2 study (NCT04252365), patients with advanced NSCLC without EGFR or ALK alterations were randomized (1:1) to receive sintilimab or pembrolizumab monotherapy (PD-L1 TPS ≥50%), or sintilimab or pembrolizumab plus platinum-based chemotherapy (PD-L1 TPS <50%). The sample size was calculated by optimal two-stage design. The primary endpoint was the objective response rate (ORR).
The study included 71 patients (sintilimab arms, n=35; pembrolizumab arms, n=36) and met its primary endpoint, with a confirmed ORR of 51.4% (18/35) in the sintilimab arms. The confirmed ORR (95% confidence interval) was 46.2% (19.2, 74.9) and 42.9% (17.7, 71.1) for patients treated with sintilimab and pembrolizumab monotherapy, and 54.5% (32.2, 75.6) and 45.5% (24.4, 67.8) for those treated with sintilimab- and pembrolizumab-based combination therapies.
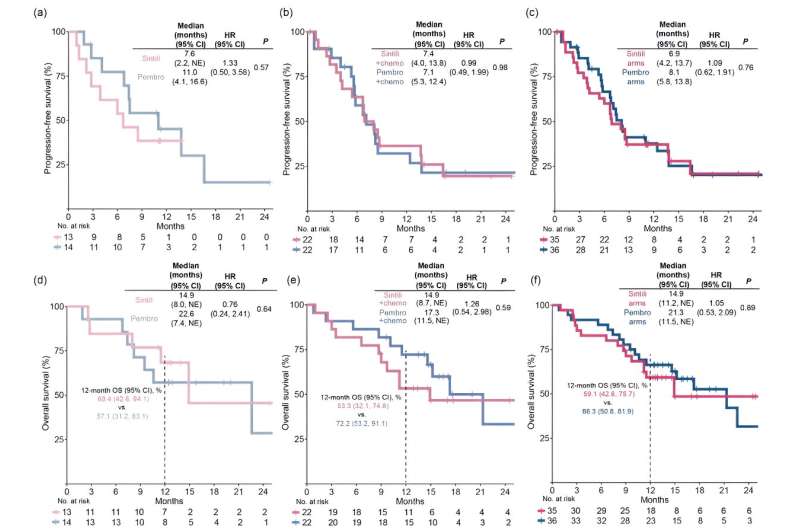
The median progression-free survival was 6.9 versus 8.1 months for all sintilimab-treated versus all pembrolizumab-treated patients, respectively, in which it was 7.6 versus 11.0 months in monotherapy and 7.4 versus 7.1 months in combination therapies.
Kaplan-Meier estimates of progression-free survival assessed by RECIST v1.1 per investigator in patients treated with (a) sintilimab versus pembrolizumab as monotherapy, (b) sintilimab versus pembrolizumab plus platinum-based doublet chemotherapy, respectively, as combination therapy, and (c) sintilimab versus pembrolizumab arms including patients who received mono- and combination therapy. Kaplan-Meier estimates of overall survival in monotherapy (d), combination therapy (e), and sintilimab versus pembrolizumab arms (f). Credit: Science China Press
The median overall survival was 14.9 versus 21.3 months for all sintilimab-treated versus all pembrolizumab-treated patients, respectively, in which it was 14.9 versus 22.6 months in monotherapy and 14.7 versus 17.3 months in combination therapies. Treatment-related adverse events were consistent with the safety outcomes of monotherapy and combination therapy in previous phase III studies. However, the incidence of rash was higher with sintilimab than with pembrolizumab monotherapy.
This is the first prospective phase 2 study to directly compare two anti-PD-1 antibodies as first-line treatment in advanced NSCLC. Sintilimab was efficacious and well-tolerated irrespective of PD-L1 expression level in patients with advanced NSCLC and had similar efficacy and safety to pembrolizumab.
More information: Si-Yang Maggie Liu et al, PD-L1 expression guidance on sintilimab versus pembrolizumab with or without platinum-doublet chemotherapy in untreated patients with advanced non-small cell lung cancer (CTONG1901): A phase 2, randomized, controlled trial, Science Bulletin (2023). DOI: 10.1016/j.scib.2023.12.046
Provided by Science China Press