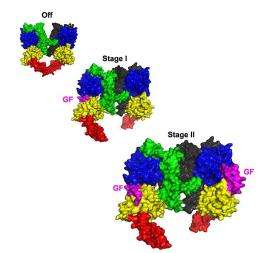
The fly EGFR as a 'two-stage switch'. Its structure is shown in three stages, left to right. At left, no growth factors are bound (off). However, as one (middle) and two (right) growth factors bind (shown in magenta) it sends two different signals
(PhysOrg.com) -- The protein EGFR, which is the target of several cancer drugs, has a split personality at the cell surface, with two different classes (high-affinity and low-affinity), whose origins have been a mystery since the 1970s. Now, a new paper by Penn researchers published in Cell explains the difference between these two classes.
Many types of tumors grow because of over-expression or a mutation of a protein called the epidermal growth factor receptor (EGFR), normally regulated by a hormone-like peptide called the epidermal growth factor (EGF). Several cancer drugs, including Herceptin, Erbitux, Iressa and Tarceva fight tumors by blocking EGFR and related receptors, notes Mark A. Lemmon, PhD, Professor and Chair of Biochemistry and Biophysics at the University of Pennsylvania School of Medicine.
EGFR has a split personality at the cell surface, with two different classes (high-affinity and low-affinity), whose origins have been a mystery since the 1970s. Now, a new paper from the Lemmon lab published recently in Cell explains the difference between these two classes by examining the structure of EGFR and its interactions with EGF.
The researchers took advantage of unique properties of the fruit fly equivalent of human EGFR as a window into the molecules' structures. They found that the EGF receptor acts like a two-stage switch.
“Two growth factors can bind to the receptor that signals across the membrane,” explains Lemmon. “With no receptors bound, the receptor is fully off. But, it looks as if there is a different type of signal if just one of the sites is filled as compared to when EGF is bound to both.” Interestingly, the insulin receptor is known to have similar properties.
Provided by University of Pennsylvania School of Medicine