Sleep spindles during NREM sleep in CaV3.1+/+ (WT) and CaV3.1−/− (KO) mice. (A) Sample traces show the raw (upper trace) and filtered (lower trace) EEG signals recorded during NREM sleep. Bandpass-filtered (6–15 Hz) EEG signals clearly show spindle events (arrowheads) in both genotypes. (B) There were no differences in the mean length of each spindle episode, number of episodes, mean peak-to-peak amplitude, and peak frequency between CaV3.1+/+ (WT) and CaV3.1−/− mice. Copyright © PNAS, doi:10.1073/pnas.1320572110
(Medical Xpress)—A recent neurological addressing one of the most fundamental issues in sleep rhythm generation study underscores an inconvenient truth—namely, that established scientific facts have and will continue to change. Researchers at Institute for Basic Science (Daejeon), Korea Institute of Science and Technology (Seoul) and Yonsei University (Seoul) have demonstrated significant exceptions to the theory, long accepted as dogma, that low-threshold burst firing mediated by T-type Ca2+ channels in thalamocortical neurons is the key component for sleep spindles. (A T-type Ca2+channel is a type of voltage-gated ion channel that displays selective permeability to calcium ions with a transient length of activation. Burst firing refers to periods of rapid neural spiking followed by quiescent, silent, periods. Sleep spindles are bursts of oscillatory brain activity visible on an EEG that occurs during non-rapid eye movement stage 2, or NREM-2, sleep, during which no eye movement occurs, and dreaming is very rare.) The scientists presented both in vivo and in vitro evidence that sleep spindles are generated normally in the absence of T-type channels and burst firing (periods of rapid neural spiking followed by quiescent, silent, periods) in thalamocortical neurons. Moreover, their results show what they describe as a potentially important role of tonic (constant) firing in this rhythm generation. They conclude that future studies should be aimed at investigating the detailed mechanism through which each type of thalamocortical oscillation is generated.
Dr. Hee-Sup Shin and Prof. Eunji Cheong discussed the paper that they recently published in Proceedings of the National Academy of Sciences. "The previous theory implicated thalamocortical TC burst firing in all sleep waves which appear in different sleep stages," Cheong tells Medical Xpress. "However, we've long questioned the extent to which thalamocortical T-type Ca2+ channels and the resulting burst firing contribute to the heterogeneity of thalamocortical oscillations during non-rapid eye movement sleep consisting of multiple brain waves." A T-type Ca2+channel is a type of voltage-gated ion channel which displays selective permeability to calcium ions, in this case with a transient length of activation.
Shin notes that the scientists faced a number of issues in designing and interpreting the results of the in vivo and in vitro experiments to test their hypothesis. "Since we observed the quite intact sleep spindles in CaV3.1 knockout mice, we tried to figure out how the sleep spindles are generated in the absence of a thalamocortical burst." (A gene knockout, or KO, is a genetic technique in which one of an organism's genes is made inoperative to learn about its function from the difference between the knockout organism and normal individuals. CaV3.1 is a T-type calcium channel found in neurons, cells that have pacemaker activity.) "The issues were if the spindles are generated within the thalamocortical circuit as previously known, and how thalamocortical neurons generate spikes during spindles in the presence or absence of a thalamocortical burst." All of the researchers' the experiments were designed to investigate these questions.
"The purpose of in vitro thalamocortical-thalamic reticular nucleus," or TC-TRN, "network oscillations was to show if thalamocortical oscillations observed in CaV3.1 knockout mice could be generated either within an intrathalamic network or if they were cortical driven oscillations," Cheong points out. "Another difference between in vivo and in vitro networks is that compared to in vivo network all the afferent inputs into TC or TRN are not intact in an in vitro TC-TRN network." The results showed that spindle-like oscillations were generated even in the absence of cortex.
The study shows that these differences also relate to In vivo data suggesting that TRN neurons are spindle pacemakers. "There have been debates on the leading role of TRN versus cortex in pacing the sleep spindles. In an in vitro TC-TRN network, both the afferent inputs and corticothalamic inputs onto TC neurons are not intact," Shin explains. "Therefore, major inputs onto TC neurons in those experiments come from TRN neurons. The generation of intrathalamic oscillations under this condition indicates that the reciprocal connection between TRN and TC could generate the oscillations, which adds weight to the TRN neurons as spindle pacemakers. The generation of CaV3.1 knockout mice which lack T-type Ca2+ channels in TC neurons was the key to address this issue."
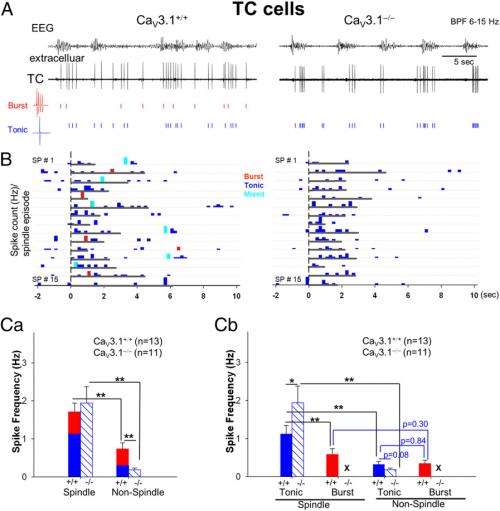
Cheong emphasizes that the study's major findings call into question the essential role of low-threshold burst firings in thalamocortical neurons. "It's noteworthy that tonic spikes were more abundant than burst spikes during spindles even in wild Type thalamocortical neurons – not only in CaV3.1-/- TC neurons – whereas no difference in tonic and burst spike frequency was seen during non-spindle periods. Moreover," he continues, "the tonic spike frequency increases significantly during cortical spindle events compared to non-spindle periods even in wild-type TC neurons. This is clearly different from that seen for burst spike frequency in wild-type TC neurons, which occurred with almost equal incidence during both the spindle and non-spindle periods." Therefore, Cheong points out, the scientists concluded that TC burst firing is not required for the generation in spindle generation.
The researchers also found that the peak frequency of sleep spindles was not different between wild and CaV3.1 KO mice, which suggested that TC spikes are not critical in determining the spindle frequency. However, Shin notes, the question of what drives TC neurons to fire during spindles remains to be further investigated, although they think that TC firing during spindles indicates that the TC-TRN network is not as simple as previously believed.
Moving forward, Cheong tells Medical Xpress, the researchers would like to further investigate the firing pattern of TC neurons during natural NREM sleep, including spindle, delta and slow waves. and also elucidate the detailed ensemble behavior of neuron within thalamocortical network during sleep. Moreover, TC burst firing has long been implicated in both physiological thalamocortical oscillations during both sleep and pathological thalamocortical oscillations, such as spike-wave-discharges appearing in absence epilepsy. "Our current study clearly showed that TC burst are not essential for sleep spindles, which would be helpful information to develop the anti-epileptic agents," Shin concludes.
More information: Sleep spindles are generated in the absence of T-type calcium channel-mediated low-threshold burst firing of thalamocortical neurons, PNAS December 10, 2013 vol. 110 no. 50 20266-20271, doi:10.1073/pnas.1320572110
Journal information: Proceedings of the National Academy of Sciences
© 2013 Medical Xpress. All rights reserved.