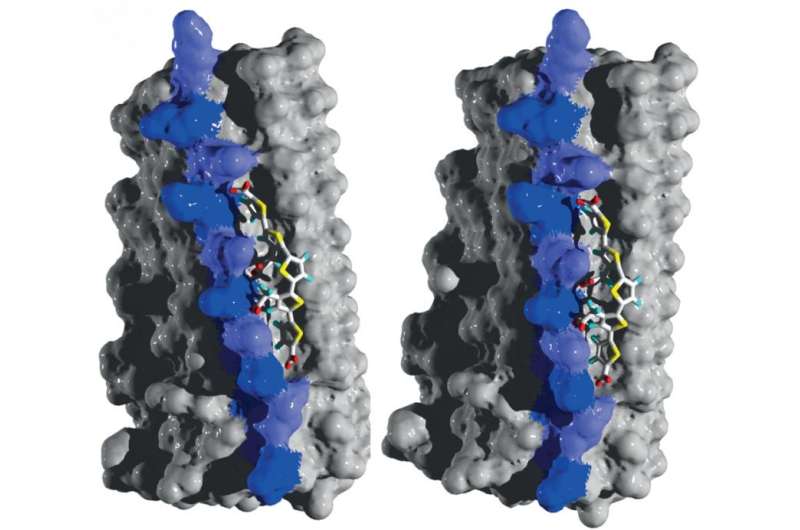
Using structural modeling, researchers conducted binding studies to study how luminescent conjugated polythiophenes (LCPs), molecules with a high affinity for protein aggregates, bind to prions. Two visualizations from different angles, illustrating the details of the interaction between LCP and amyloid, are shown. The two key residues for binding are marked in two different shades of purple. Credit: Herrmann et al., Science Translational Medicine (2015)
A new drug compound could lead to a breakthrough in the fight against bovine spongiform encephalopathy, the incurable brain-wasting disease also known as mad cow disease, researchers said Wednesday.
Scientists said the new antiprion drug, known as polythiophene, has proven highly effective in mice in blocking toxic, misfolded prions.
Prions have been linked to dementia, personality shifts and other disorders, including Creutzfeldt-Jakob disease—the human equivalent of mad cow disease.
At present, no medication has proven effective in preventing or curing those neurodegenerative ailments.
But research in the American journal Science Translational Medicine said the new anti-prion compound appears to be successful in locking infectious clumps of prions in place.
Researchers said infectious prions replicate by triggering normal forms of the protein to fold abnormally and cluster into long chains that are highly toxic to neurons.
Scientist Uli Herrmann and his research colleagues designed new polythiophenes with optimal ability to immobilize prions, the most promising of which prolonged survival of prion-infected mice and hamsters by more than 80 percent.
The mice and hamsters treated with the compound showed fewer prion clusters and less severe damage in the brain, the scientists said, suggesting that it stabilized small clusters of prions and locked them in place, preventing self-replication.
Molecular dynamics simulations show that the structural stability of the β sheet of a protein aggregate display significant fluctuations in the absence of LCP. Credit: Herrmann et al., Science Translational Medicine (2015)
The encouraging results suggest that polythiophene could prove to be a potent future treatment for prion diseases, according to the study.
Mad cow disease can be fatal to humans who eat an infected cow's meat.
Scientists believe the disease, including a major outbreak in Britain in the 1990s, was caused by using infected parts of cow to make feed for other cattle.
Experts believe eating meat from infected animals can trigger the CJD, human variant of the fatal brain-wasting malady.
Hermann is affiliated with the Institute for Neurology at the University of Zurich in Switzerland.
Another key researcher on the study was Anja Boeckmann, at the Institute for Biology and Chemical Proteins at the University of Lyon in France.
More information: Structure-based drug design identifies polythiophenes as antiprion compounds, stm.sciencemag.org/lookup/doi/ … scitranslmed.aab1923
Journal information: Science Translational Medicine
© 2015 AFP