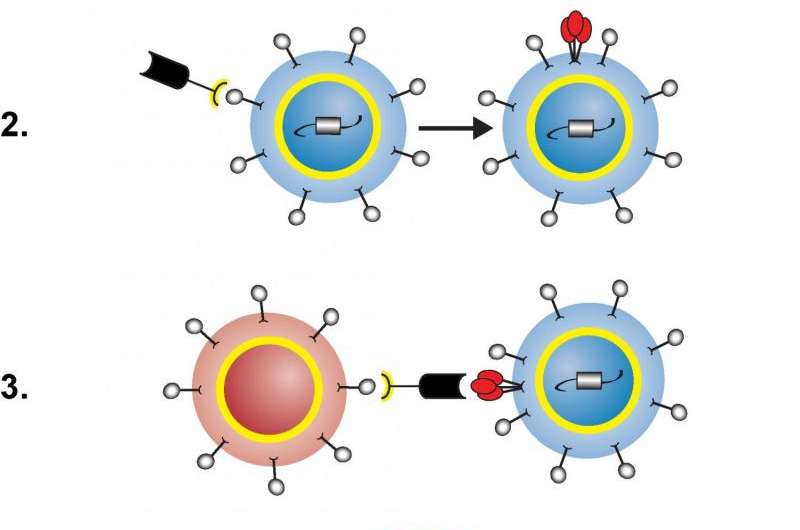
An illustration of how the engineered protein facilitates destruction of latently HIV-infected immune cells. 1) Protein and cells, from left to right: engineered protein with yellow-and-black CD3-binding end and thick black HIV-binding end; latently HIV-infected helper T cell (blue); inactivated killer T cell (red). 2) Protein binds to CD3 receptor on helper T cell, activating it so the helper T cell starts making HIV and displaying pieces of virus (red) on its surface. 3) Protein binds to HIV fragment on helper T cell and CD3 receptor on killer T cell, activating the killer T cell and bringing the two cells close together. 4) Activated killer T cell destroys HIV-infected helper T cell. Credit: NIAID
Scientists at the National Institutes of Health (NIH) have created a protein that awakens resting immune cells infected with HIV and facilitates their destruction in laboratory studies. The protein potentially could contribute to a cure for HIV infection by helping deplete the reservoir of long-lived, latently HIV-infected cells that can start making the virus when a person stops taking anti-HIV drugs. Further studies in animals and people are needed to determine the viability of this approach.
The researchers found that the protein, called VRC07-αCD3, triggered the activation and killing of latently HIV-infected helper T cells when the cells were taken from patients on antiretroviral therapy and then incubated in the lab with the patients' own killer T cells. In addition, the scientists found a monkey-adapted version of the protein to be safe and well-tolerated when given to monkeys infected with a simian form of HIV and receiving antiretroviral therapy. The researchers are now studying the effectiveness of monkey-adapted VRC07-αCD3 in the animals.
The engineered protein has two ends: one activates T cells by binding to a surface molecule called the CD3 receptor, and the other—based on an antibody called VRC07—powerfully binds to more than 90 percent of HIV strains. VRC07-αCD3 facilitates the killing of latently HIV-infected cells in three steps. First, the CD3-binding end attaches to a resting, HIV-infected helper T cell, activating the cell so it starts making HIV and displaying pieces of virus on its surface. Next, the HIV-binding end of the protein latches onto those pieces of virus while the CD3-binding end attaches to a killer T cell, activating it and bringing it close to the helper T cell. Finally, the activated killer T cell destroys the HIV-infected helper T cell.
A team of scientists at the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases, part of NIH, created VRC07-αCD3 under the leadership of VRC Director John R. Mascola, M.D.; former VRC Director Gary J. Nabel, M.D., Ph.D.; and Richard A. Koup, M.D., VRC deputy director and chief of its immunology laboratory.
More information: Amarendra Pegu et al. Activation and lysis of human CD4 cells latently infected with HIV-1, Nature Communications (2015). DOI: 10.1038/ncomms9447
Journal information: Nature Communications