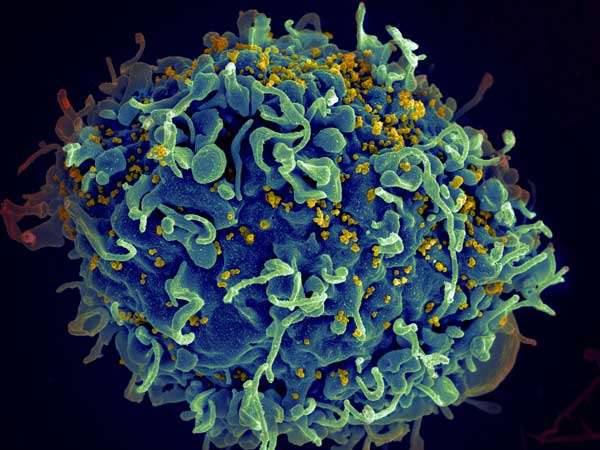
HIV (yellow) infecting a human immune cell. Credit: Seth Pincus, Elizabeth Fischer and Austin Athman, National Institute of Allergy and Infectious Diseases, National Institutes of Health
After nearly three decades of battling the HIV virus, Frank Brooks is daring to believe what once seemed unthinkable: A life free of AIDS.
"That is what I'm living for," said Brooks, 70. "I am optimistic, if I hang on, I will be here for that cure."
Long-term survivors like Brooks were buoyed by news Tuesday at the first-ever HIV Cure Summit, held on World AIDS Day by scientists at University of California, San Francisco, focusing on three major strategies to vanquish a virus that has killed about 40 million people worldwide.
"We are in the forefront of the crucial final chapter of the HIV epidemic," said Dr. Paul Volberding, a UCSF professor of medicine who treated some of the epidemic's first victims in the early 1980s.
Only one person has ever been cured of the virus. That was the so-called "Berlin patient", now identified as Timothy Ray Brown, an American treated in Germany with gene therapy. The UCSF teams vow to build a scientific basis for a more widespread cure within five years, "an audacious but achievable goal," said UCSF Chancellor Sam Hawgood.
While drug treatment is helping prevent infections and stabilizing the health of those who are already sick, it is expensive and not available to people in developing countries.
And as soon as treatment ends, the virus flares up.
That's been the huge hurdle to a cure: HIV hides inside our immune cells - unreachable by current drugs and unrecognizable to the immune system.
Since the beginning of the epidemic in the early 1980s, more than 96,000 Californians diagnosed with AIDS have died, according to the San Francisco AIDS Foundation. Now, more than 35 million people worldwide are infected with HIV, including thousands of Bay Area residents living with the virus.
But insights into basic biology and immunology have identified three major strategies to cure them. All are now being tested in animals, and will start moving into the clinic to help patients. They are:
-Gene therapy, which takes an HIV-infected person's own cells and knocks out or disables a gene, known as CCR5, that acts as a viral doorway.
-Vaccination, which doesn't kill the virus but builds up the patient's immune system to prevent it from spreading.
-"Shock and kill," which uses a "shock" drug to find and activate latent HIV, then kills it as soon as it emerges.
Envision an HIV cure like killing weeds in a lawn, said Rowena Johnson, director of research for amfAR, the Foundation for AIDS Research, which is funding UCSF's new $20 million Institute for HIV Cure.
Traditional anti-viral drugs act like a lawn mower, keeping weeds manicured and under control. But once the gardener leaves, weeds resurge - it doesn't matter how often you mow, the weeds will persist.
Gene therapy is akin to excavating all the soil and replacing it with new soil. This is a potentially traumatic and expensive exercise, not easily exported to developing countries, said UCSF scientists. But it may also be the best bet for a true cure. Funds from the taxpayer-supported California Institute for Regenerative Medicine is testing this approach in three different clinical trials.
Vaccination is like spreading black plastic over the lawn; while promising, nothing yet has been strong enough to kill the virus.
"Shock and kill," believed to be the most practical strategy, is like adding fertilizer to trigger growth-then killing the entire plant, roots and all, with Roundup.
The first step to "shock and kill" - the main focus of the UCSF teams - is to find exactly where in the tissues the latent HIV is lingering, using newly designed tools. This has been a needle-in-the-haystack challenge, since only one in 1 million immune cells are infected.
The UCSF scientists will then aim to administer a drug called a "shocking agent," which stimulates the virus to emerge from its hiding place, then kill it, using another agent to awake and deploy the immune system.
This strategy has yielded promising results in monkeys, reducing the amount of hidden virus; now, scientists hope to extinguish it.
"We need a good kill strategy to remove every last reservoir cell," said Greene. "Multiple rounds of shock will be required."
As long as the virus is still hiding in the cells, patients will suffer from symptoms, said Satish Phillai of the UCSF Blood Systems Research Institute. "Little bits and pieces of virus left can create chaos."
The best we may do is a "functional cure," where HIV is still present but levels are so well suppressed that little or no medication is needed, they said.
Alternatively, patients may stay in "remission" - not needing medication but needing constant monitoring in case the virus suddenly reappears.
The dream is what's called a "sterilizing cure," where it's gone forever.
"Time is not on my side," said Brooks. "But I never thought I'd live to see my 50th birthday, and I just celebrated my 70th."
"We are light years ahead of where we were in the 1980s, when it was so terrifying. People were dying left and right."
"Now we understand what is left to do," he said. "And how to get there.."
©2015 San Jose Mercury News (San Jose, Calif.)
Distributed by Tribune Content Agency, LLC.