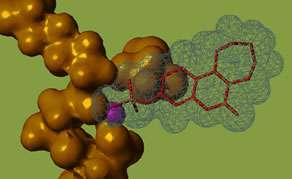
Irosustat (red) binding to its drug target. Credit: University of Bath
New clinical data were revealed at the American Society for Clinical Oncology (ASCO) in Chicago last week, the world's largest cancer congress, for Irosustat, a drug pioneered at the University of Bath that could prolong the lives of women with breast cancer.
As one of the most common cancers, breast cancer represents a large unmet medical need, with more than 50,000 new cases diagnosed in the UK every year.
The orally active drug, Irosustat, was first designed and chemically synthesised in the Department of Pharmacy & Pharmacology. Irosustat formed part of the assets of the Bath-Imperial College spin-out company Sterix Ltd that was co-founded by Professor Barry Potter and was acquired by the pharmaceutical company Ipsen in 2004.
New clinical trials
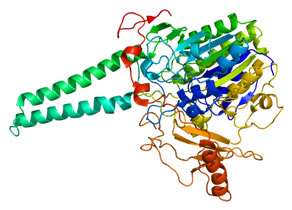
Irosustat is a 'first-in-class' inhibitor of the enzyme steroid sulfatase, designed to block the production of oestrogen, which fuels cancer development in two-thirds of all cases. It has already been in numerous international clinical trials in both men and women. The new data relate to a Phase II study in postmenopausal breast cancer in combination with a well-established clinical therapy, a so-called "aromatase inhibitor", that also blocks the production of oestrogen by a different pathway.
Currently, women who have had surgery for breast cancer are often prescribed aromatase inhibitors for five years to prevent tumours returning. Aromatase inhibitors made worldwide news at ASCO last week with a major study on 2000 women showing that taking them for an extra five years greatly increased protective effects. Women were 34 per cent less likely to have a recurrence and a fifth less at risk of dying.
Drug target steroid sulfatase. Credit: University of Bath
The new clinical trial, the IRIS study, carried out at nine UK NHS Trusts, led by Imperial College Clinical Trials Unit and supported by CRUK, investigated whether adding Irosustat to an aromatase inhibitor can further reduce oestrogen and control the cancer better. 27 Postmenopausal women with locally advanced or metastatic breast cancer not controlled by aromatase inhibitor treatment received Irosustat once daily in addition to the aromatase inhibitor on which they were progressing.
When added to an aromatase inhibitor Irosustat was well-tolerated and resulted in clinical benefit in cancer patients, stable disease and added progression-free survival. These data validate the underpinning science and provide exciting evidence that blocking steroid sulfatase in combination in such advanced breast cancer settings can result in clinical activity. A second trial IPET using Irosustat alone also showed responses and is the first to demonstrate clinical activity of the drug in early breast cancer.
Novel approach for hormone-responsive breast cancer
Professor Potter's group is already pursuing a novel approach for hormone-responsive breast cancer with the design of dual aromatase-sulfatase inhibitors in a single drug molecule and has already shown that the concept will work. The present clinical data support this approach and the aim is to translate such a new agent rapidly into clinical trials.
Commenting on the developments Professor Potter said: 'The most rewarding thing for any Medicinal Chemist and Drug Discovery Group is to see a drug synthesized in the lab overcome the multitude of hugely difficult hurdles and finally be administered in clinical trials to patients in need. This requires considerable diverse teamwork, comes at a multi £M cost and is an extremely rare event in academia, as opposed to the pharmaceutical industry.
'Only the tiniest fraction of all such development candidates ever make it this far in any setting. I am truly delighted that our approach is translating into continuing real clinical benefit for cancer patients. Larger studies are now needed for this drug.'
Provided by University of Bath