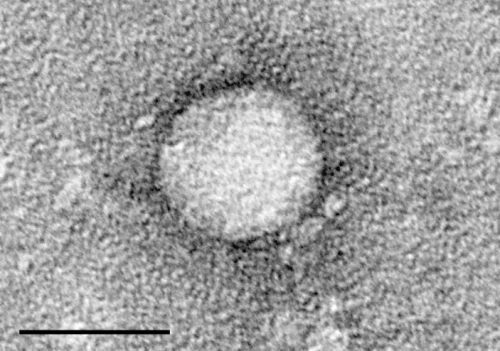
Electron micrographs of hepatitis C virus purified from cell culture. Scale bar is 50 nanometers. Credit: Center for the Study of Hepatitis C, The Rockefeller University.
FDA-approved oral direct-acting antiviral (DAA) regimens produce high sustained virologic response (SVR) rates for all six hepatitis C virus (HCV) genotypes and for patient populations historically considered difficult to cure. The ease of dosing, safety profile, and effectiveness of DAAs provide an opportunity to reduce the burden of hepatitis C in the United States, provided barriers to care are addressed.
The results of a systematic evidence review are published in Annals of Internal Medicine.
Researchers at Johns Hopkins University School of Medicine in Baltimore, MD, reviewed data from 42 published clinical trials of adults with chronic HCV infection that evaluated at least 8 weeks of an interferon-free HCV regimen that included at least 2 FDA-approved DAAs. These included inhibitors of HCV NS3 protease (grazoprevir, paritaprevir, and simeprevir); NS5A (daclatasvir, elbasvir, ledipasvir, ombitasvir, and velpatasvir); and NS5B polymerase (sofosbuvir and dasabuvir), as well as the oral antiviral ribavirin. Six DAA regimens showed SVR rates of greater than 95 percent in patients with HCV genotype 1 without cirrhosis, including those with HIV co-infection. Cure rates were similar for the remaining genotypes of HCV, although fewer regimens were effective for genotype 2.
The authors of an accompanying editorial suggest that these findings provide hope that HCV is now fully treatable and can potentially be eliminated as an important medical problem in the United States. While HCV is now easy to treat, there are still some issues, such as cost and access to care, which need to be addressed.
More information: Annals of Internal Medicine, http://annals.org/aim/article/doi/10.7326/M16-2575
Journal information: Annals of Internal Medicine
Provided by American College of Physicians