Abbott Laboratories has gained clearance to start selling in the U.S. the first continuous glucose monitor that does not require people with diabetes to routinely prick their fingers.
The U.S. Food and Drug Administration recently approved Abbott's FreeStyle Libre Flash Glucose Monitoring System for adults, which already is sold in 41 other countries.
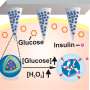
The device consists of a small sensor, about the size of a quarter, that's worn on the back of the upper arm to continuously track glucose levels. The sensor, unlike other wearable sensors, does not require patients to prick their fingers for calibration. Patients can place a hand-held reader near the device to see their current glucose levels, trends, patterns and where those levels might be headed. They can then use those readings to figure out how much insulin to take to manage their diabetes.
The device has not yet been approved for use by children in the U.S. but Abbott hopes to gain approval from the FDA.
The company is not disclosing pricing information until it gets closer to launching the product in the U.S., which will likely be before the end of the year, said Abbott spokeswoman Vicky Assardo. But she said in an email the price will be "very similar" to the price in Europe, where the reader costs about $69, and each sensor, which lasts about 14 days, also costs about $69, before insurance. In the U.S., the sensor will last about 10 days.
"We intentionally designed the product to make it as affordable as possible," said Jared Watkin, Abbott senior vice president of diabetes care.
In the U.S., continuous glucose monitors are often covered by insurance, Watkin said. He said Abbott plans to have discussions about coverage with insurers in the U.S. and is confident it will be able to show them the advantages of covering it.
Regional and national health insurance systems in 18 countries - including the United Kingdom, France, Germany and Japan - already have agreed to fully or partially cover the devices.
Many consumers are eager for the device to finally hit the market in the U.S. Melissa Polovin, of Deerfield, Ill., said she's been waiting two years for the FDA approval, ever since she took part in a clinical trial in which she helped test a similar device made by Abbott. "I am champing at the bit for this thing," said Polovin, who was diagnosed with Type 1 diabetes in 1994.
Now she typically pricks her finger about four times a day to get blood sugar readings, she said. But it's not the pain of the finger sticks that bothers her.
"There's so much management and so much maintenance and anything that feels simpler and less invasive ... it's a world of difference," she said.
Abbey Studer, of Edgewater, in Chicago, also sticks her finger about four to six times a day. She often wears a continuous glucose monitor made by another company that tells her insulin pump how much basal insulin she needs throughout the day.
She said those pricks don't hurt anymore because of callouses on her fingers, but they can be an intrusion on her daily life. "I think the psychological impact of being asked to do something, to calibrate at an inconvenient time, in the middle of the night, in the middle of a meeting, when you're trying to breastfeed your child - that aspect changes your life," Studer said.
Studer is cautiously optimistic about the new Abbott device. The device doesn't necessarily eliminate all finger pricks for people with diabetes. Patients may still have to stick their fingers to confirm the device's readings if they get particularly low or rapidly changing readings or when their symptoms don't match glucose readings, said Mahmood Kazemi, Abbott senior director of global medical and scientific affairs.
The device also does not alert patients of low glucose levels without a patient waving the reader over his arm.
Also, the system doesn't communicate with an insulin pump as other continuous glucose monitors do - though Abbott is working with a Silicon Valley-based company on that feature.
Dr. Rasa Kazlauskaite, director of the diabetes technology Initiative at Rush University Medical Center, said the new device could be an exciting development for patients, but she cautioned that, ultimately, its usefulness will depend on its accuracy. Kazlauskaite is not working with Abbott.
She's a big fan of continuous glucose monitors in general, calling them the "second greatest invention" in diabetes management after the invention of insulin injections. But Kazlauskaite said continuous glucose monitors can sometimes give inaccurate readings, necessitating traditional finger sticks. "No technology is entirely perfect," she said.
The FDA warned in a news release that patients could experience abnormally high or low glucose levels in cases where information provided by the device is inaccurate, and that information is used to make treatment decisions.
Abbott's Kazemi, however, said Abbott is confident in the device's accuracy. Patients using the devices check their glucose levels an average of 16 times a day in Europe, where the devices already are available, he said.
"It allows people to finally do what we've been asking them to do all along," he said, referring to asking patients to regularly check their glucose levels.
©2017 Chicago Tribune
Distributed by Tribune Content Agency, LLC.