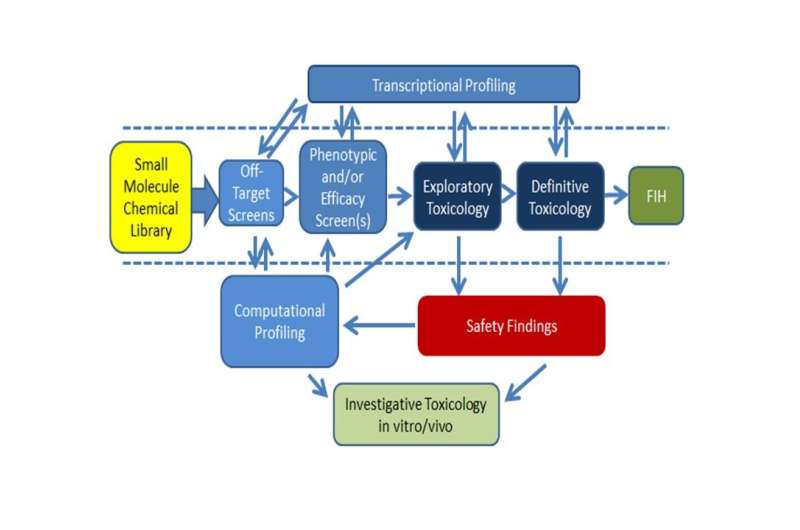
Example of a drug development paradigm incorporating multi-step screening and showing relationships between data that can be integrated to combine complimentary data sets that provide a more complete picture of drug-related effects. Credit: Van Vleet et al. (AbbVie, Chicago, IL USA)
A new review in SLAS Discovery explores how improved safety screening strategies and methods are improving the pharmaceutical discovery and development process. Terry R. Van Vleet et al. of AbbVie (Chicago, IL USA) outline several fundamental methods of the current drug screening processes and emerging techniques and technologies that promise to improve molecule selection. In addition, the authors discuss integrated screening strategies and provide examples of advanced screening paradigms.
Pharmaceutical discovery and development is a long and expensive process that, unfortunately, still results in a low success rate, with drug safety continuing to be a major impedance. Improved safety screening strategies and methods are needed to more effectively fill this critical gap.
Recent advances in informatics are now making it possible to manage bigger data sets and integrate multiple sources of screening data in a manner that can potentially improve the selection of higher-quality drug candidates. Integrated screening paradigms have become the norm in pharma, both in discovery screening and in the identification of off-target toxicity mechanisms during later-stage development.
Furthermore, advances in computational methods are making in silico screens more relevant and suggest that they may represent a feasible option for augmenting the current screening paradigm.
More information: Terry R. Van Vleet et al, Screening Strategies and Methods for Better Off-Target Liability Prediction and Identification of Small-Molecule Pharmaceuticals, SLAS DISCOVERY: Advancing Life Sciences R&D (2018). DOI: 10.1177/2472555218799713
Provided by SLAS (Society for Laboratory Automation and Screening)