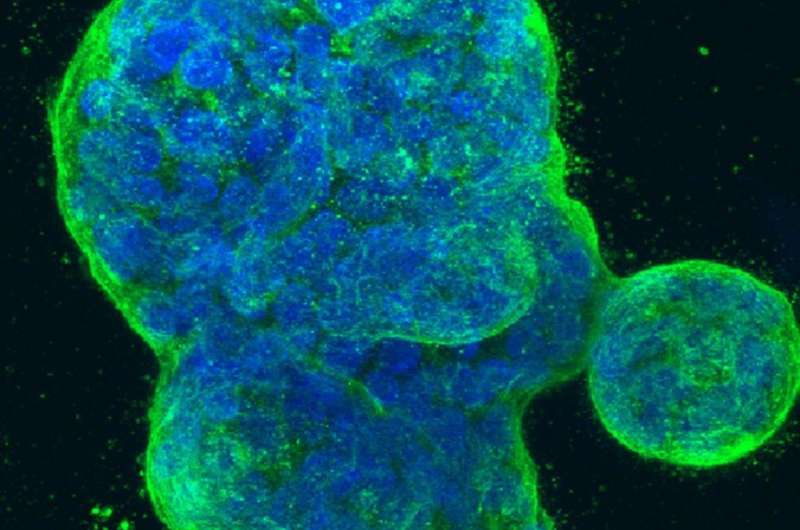
Three-dimensional culture of human breast cancer cells, with DNA stained blue and a protein in the cell surface membrane stained green. Image created in 2014 by Tom Misteli, Ph.D., and Karen Meaburn, Ph.D. at the NIH IRP.
Immunotherapies have transformed cancer care, but their successes have been limited for reasons that are both complex and perplexing.
In breast cancer especially, only a small number of patients are even eligible to undergo treatment with immunotherapies, and most see little benefit.
But in a pre-clinical study led by the Duke Cancer Institute, researchers outlined a potential way to improve those results by uncloaking breast cancer tumors to the body's immune system.
Publishing this month in the journal Nature Communications, the researchers identified an enzyme in cells involved in regulating the growth and spread of breast cancers. Testing in mice, they demonstrated a way to shut down the enzyme's activity to allow T-cells to mount an immune attack.
"We found that inhibition of the activity of this enzyme decreased the ability of macrophages in tumors to suppress an immune attack on cancer cells and indeed encouraged them to start producing chemicals that attract more cancer-killing T cells into the tumor," said Donald McDonnell, Ph.D., chair of Duke's Department of Pharmacology & Cancer Biology. "We can basically uncloak the tumor to the immune system."
McDonnell and colleagues, including lead author and collaborator Luigi Racioppi, M.D., Ph.D., reported that a kinase, or enzyme, called CaMKK2 is highly expressed in macrophages within human breast tumors. They performed a series of exploratory studies that revealed the molecule's potential utility as a therapeutic target for breast cancer. Working with colleagues at the University of North Carolina at Chapel Hill, they developed a new class of drugs that inhibited the growth of human breast tumors grown in mice.
"The use of this molecule suppressed tumor growth not only by increasing the accumulation of tumor-killing T cells, but also by reducing the tumor's capability to suppress T cell activity," McDonnell said. "It's solving two problems, like we couldn't get into the bar, and if we did, we couldn't get a drink. Now we can do both."
McDonnell said additional studies are ongoing, with the goal of acquiring data to launch a clinical trial in breast cancer patients within the next 18 months.
More information: Luigi Racioppi et al. CaMKK2 in myeloid cells is a key regulator of the immune-suppressive microenvironment in breast cancer, Nature Communications (2019). DOI: 10.1038/s41467-019-10424-5
Journal information: Nature Communications
Provided by Duke University Medical Center