(HealthDay)—For high-risk outpatients with COVID-19, fluvoxamine reduces the need for hospitalization, according to a study published online Oct. 27 in The Lancet Global Health.
Gilmar Reis, Ph.D., from the Pontificia Universidade Católica de Minas Gerais in Belo Horizonte, Brazil, and colleagues recruited from 11 clinical sites high-risk symptomatic Brazilian adults who were confirmed positive for severe acute respiratory syndrome coronavirus-2 and had a known risk factor for progression to severe disease. Participants were randomly assigned to fluvoxamine (100 mg twice daily for 10 days) or placebo (741 and 756 patients, respectively).
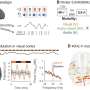
The trial arms were stopped for superiority with randomization to fluvoxamine reported from Jan. 20 to Aug. 5, 2021. The researchers found that compared with the placebo group, the fluvoxamine group had a lower proportion of patients in a COVID-19 emergency setting for more than six hours or transferred to a tertiary hospital due to COVID-19 (11 versus 16 percent; relative risk, 0.68), with a 99.8 percent probability of superiority, surpassing the prespecified superiority threshold of 97.6 percent. In the per-protocol population, there was one death reported in the fluvoxamine group and 12 in the placebo group (odds ratio, 0.09). The number of treatment-emergent adverse events did not differ significantly for patients in the fluvoxamine and placebo groups.
"Our results are consistent with earlier, smaller trials," Reis said in a statement. "Given fluvoxamine's safety, tolerability, ease of use, low cost, and widespread availability, these findings may have an important influence on national and international guidelines on clinical management of COVID-19."
Several authors disclosed financial ties to the life science industry; two of the authors are coinventors on a patent application for methods of treating COVID-19.
More information:
Abstract/Full Text
Editorial
Journal information: The Lancet Global Health
Copyright © 2021 HealthDay. All rights reserved.