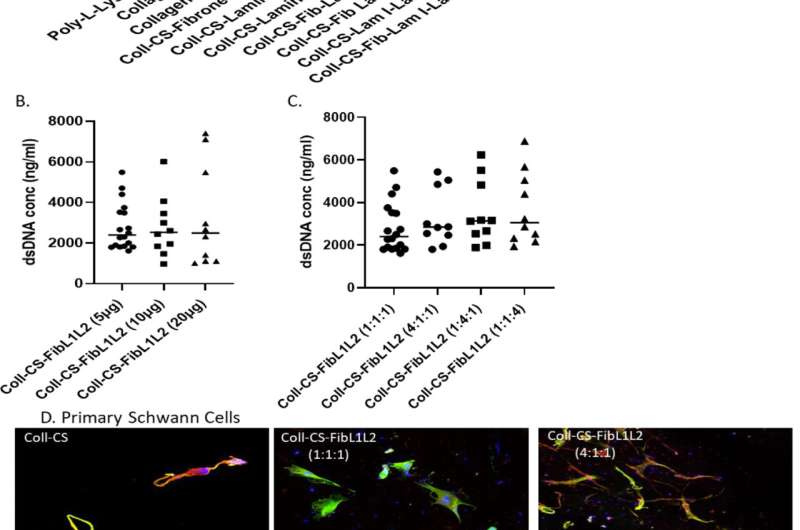
Figure 1. 2D validation of ECM substrate combinations and ratios. A) Significant changes in proliferation via dsDNA content in S42 Schwann cells at day 7 using a variety of ECM coatings compared to plates coated with 50 µg/ml of collagen I only B) No significant differences were observed in proliferation when the concentration of FibL1L2 was increased to 10 µg/ml and 20 µg/ml compared to CS-FibL1L2 at 5 µg/ml C) No significant differences were observed in proliferation when the ratios of FibL1L2 were altered to investigate the effect of an increased concentration of any one component relative to the others. D) Staining for cell nuclei (blue), s100b (green) and βIII- tubulin (red) using primary Schwann cell revealed visible differences in levels of protein expression. (n=3, 1-way ANOVA *P ≤0.05, **P ≤0.01, ***P ≤0.001). Credit: DOI: 10.1016/j.matbio.2022.01.002
Researchers from the RCSI University of Medicine and Health Sciences, AMBER, the SFI Research Centre for Advanced Materials and BioEngineering Research along with leading global medical technology company Integra LifeSciences, today announced new breakthrough for nerve repair therapies based on body's own processes in the journal Matrix Biology.
The pre-clinical study showed that use of extracellular matrix (ECM) supports improved nerve fiber regeneration across large nerve defects without the need for application of additional cells or growth factors. In these pre-clinical trials, the team's novel ECM-loaded medical device known as a 'nerve guidance conduit,' was shown to support improved recovery responses at eight weeks following the repair of traumatic nerve lacerations with substantial loss of tissue.
The research team found that by fine-tuning the combination and ratio of ECM proteins and loading them into the nerve guidance conduit, it was possible to support increased pro-repair inflammation, increased blood vessel density, and increased density of regenerating nerves, all as compared to standard of care. By mimicking the body's nerve repair processes, this new approach may eliminate the need for additional stem cells and drug therapies.
Peripheral nerve injury is a major clinical problem and is known to affect more than 5 million people worldwide every year, leaving those afflicted with loss of motor or sensory function to muscles or skin. Current therapies to repair nerve damage involve transplanting the patients' healthy nerves to repair damage or implanting an artificial nerve guidance conduit. The team's novel patented approach to nerve repair has been shown to increase the density of regenerating long-nerve structures, known as axons, and to generate a strong increase in blood vessel density to better support the regenerating tissues.
Commenting on the results, lead authors Drs. Alan Hibbitts and Zuzana Kočí from the Tissue Engineering Research Group based at Dept. of Anatomy and Regenerative Medicine at RCSI, and AMBER, said: "In our lab-based trials, we discovered that at eight weeks post implantation our nerve guidance conduit had successfully improved the prognosis for nerve regeneration and repair over the current clinical gold standard. Our conduit supported clear improvements in nerve repair and blood vessel formation and most importantly, we saw that we could scale this up to approach very large nerve defects in our pre-clinical studies."
Regarding the success of this study, Prof. Fergal O'Brien, Principal Investigator on the project and Professor of Bioengineering and Regenerative Medicine, Head of Tissue Engineering Research Group at RCSI and Deputy Director of AMBER, said the partnership between RCSI, AMBER and Integra LifeSciences was critical to ensure clinical relevance and a pathway from lab to patient.
"Working with Integra Chief Scientist, Dr. Simon Archibald, the research had a clear focus—to create a device based on scientific excellence with improved outcomes that would translate well through regulatory assessment, into the clinical setting, and ultimately, patients. This provides a more direct route to market and therefore the potential for faster real-world impact in improving patient quality of life."
Dr. Simon Archibald, Chief Scientist at Integra LifeSciences added: "We have partnered with Prof. Fergal O'Brien and his team at RCSI to innovate new solutions in regenerative medicine since 2005, and over that time, we have rapidly accelerated the development and translation of new biomaterials. We are enthusiastic for the future potential of this iterative innovation to address long-gap nerve repair, building on our current leading clinical materials for short-gap nerve repairs. Placing Integra at the coalface of research enables us to bring our expertise to the heart of the scientific process and identify clinically relevant solutions based on cutting-edge science, to improve patient outcomes and the most efficient pathway from the lab to clinical setting."
Detailing his teams' plans, Professor O'Brien said: "Our new ECM-enhanced nerve guidance conduits are part of my team's ongoing research to address long peripheral nerve defects in partnership with Integra LifeSciences. The outputs from this project will address increasingly challenging nerve defect distances with the ambition to relieve the current clinical reliance on grafted nerves and move into the next phase of pre-clinical trials. Our partnership with Integra LifeSciences has been essential to this process, and we look forward to an ambitious program of work that will advance continued enhanced treatments for nerve damage and injury."
More information: Alan J. Hibbitts et al, Multi-Factorial Nerve Guidance Conduit Engineering Improves Outcomes in Inflammation, Angiogenesis and Large Defect Nerve Repair, Matrix Biology (2022). DOI: 10.1016/j.matbio.2022.01.002
Provided by RCSI University of Medicine and Health Sciences