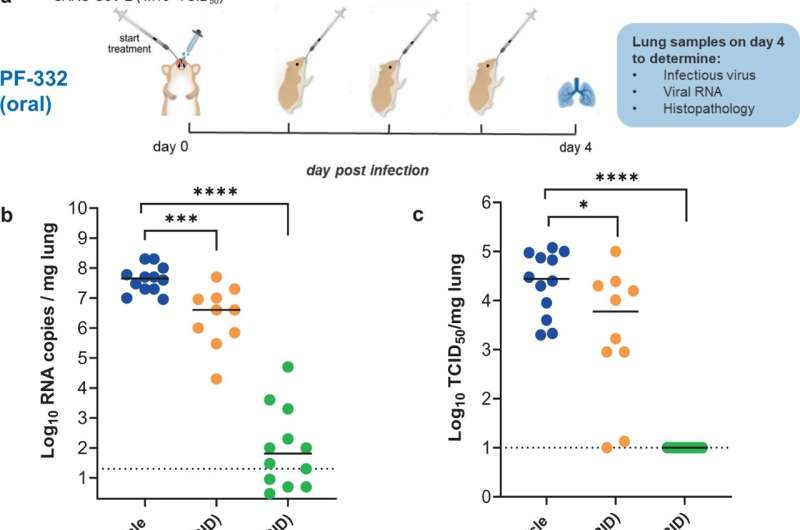
In vivo efficacy of PF-332 against Beta SARS-CoV-2 (B.1.351) variant in Syrian hamsters. a Design of the study. b Viral RNA levels in the lungs of control (vehicle-treated) and PF-332-treated (at 125 or 250 mg/kg, BID) SARS-CoV-2−infected hamsters at day 4 post-infection. Individual data and median values (indicated by bars) are presented and are expressed as log10 SARS-CoV-2 RNA copies per mg lung tissue. Data were analyzed with the Mann–Whitney U test (two-sided). ***P = 0.0007, ****P < 0.0001. c Infectious viral loads in the lungs of control (vehicle-treated) and PF-332-treated SARS-CoV-2-infected hamsters at day 4 pi (expressed as log10 TCID50 per mg lung tissue). Individual data and median values (indicated by bars) are presented. Data were analyzed with the Mann–Whitney U test (two-sided). *P = 0.034, ****P < 0.0001. d Weight change at day 4 pi in percentage, normalized to the body weight at the day of infection. Bars represent means ± SD. Data were analyzed with the Mann–Whitney U test (two-sided). **P = 0.0031, ***P = 0.0003. e Cumulative severity score from H&E stained slides of lungs from control (vehicle-treated) and PF-332-treated hamsters. Individual data and median values (indicated by bars) are presented and the dotted line represents the median score of untreated non-infected hamsters. Data were analyzed with the Mann–Whitney U test (two-sided). ****P < 0.0001. All data b–e are from two independent experiments with n = 12 for vehicle and 250 mg/kg BID groups and n = 10 for 125 mg/kg BID group. PF-332 = PF-07321332. Source data are provided as a Source Data file. Credit: DOI: 10.1038/s41467-022-28354-0
The SARS-CoV-2 anti-viral drug, PF-07321332, can protect Syrian hamsters against infection with some SARS-CoV-2 variants of concern and reduces the risk of transmission, suggests a study published in Nature Communications.
There is an urgent need for safe and effective anti-viral drugs to treat SARS-CoV-2 infections. The SARS-CoV-2 main protease, an enzyme that aids virus replication, has been identified as a promising anti-viral target. Several main protease inhibitors have shown anti-viral activity in cell culture models and animal models for both SARS-CoV and SARS-CoV-2.
To test the anti-viral efficacy of PF-07321332 (PF-332) Johan Neyts and colleagues conducted experiments in cell models and an animal model with the Alpha, Beta and Delta variants of SARS-Cov-2. As a proof-of-concept, the authors first conducted experiments in mammalian cells and a primary human airway epithelial cell model to study SARS-CoV-2 virus infection and test anti-viral compounds. They confirmed PF-332 anti-viral activity against the Alpha variant. The authors then tested the efficacy of PF-332 in small groups of Syrian golden hamsters. They found that Syrian hamsters infected via the nose with the Beta or Delta variants did not develop symptoms or show signs of illness following oral treatment with PF-332 for four consecutive days. The authors also infected six hamsters with the Delta variant and co-housed them with six uninfected animals. They found that hamsters treated with PF-332 for three days did not transmit the Delta variant to the co-housed untreated animals.
PF-332 has received emergency approval for use in some countries and the authors suggest these findings underscore the potential of PF-332 as a promising anti-viral drug to treat SARS-CoV-2 infection, limit transmission and improve disease outcome.
More information: Rana Abdelnabi et al, The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern, Nature Communications (2022). DOI: 10.1038/s41467-022-28354-0
Journal information: Nature Communications
Provided by Nature Publishing Group